News & Media
Home / News & Media
News & Media
Masimo News & Media
Stay up to date with Masimo
Masimo Consumer Audio News & Media
Stay up to date with Masimo Consumer Audio
NEWS & MEDIA:

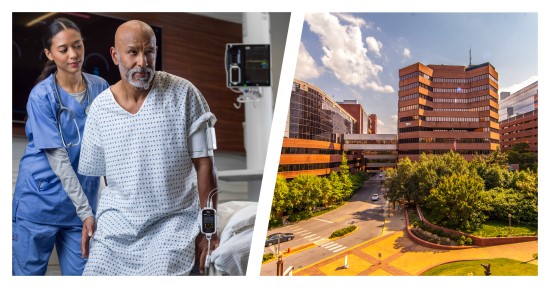
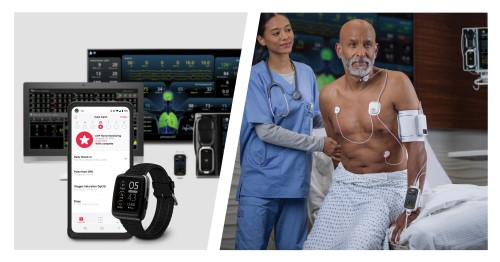
Vanderbilt University Medical Center Integrates Masimo Radius VSM™ in Proactive Care Protocol to Combat Emergency Room Congestion
Renowned Healthcare Facility Launches Advanced Wireless Monitoring Technology in the Emergency Department, Reducing Wait Times and Improving Patient Prioritization
Masimo (NASDAQ: MASI) today announced that Vanderbilt University Medical Center (VUMC), a renowned healthcare facility in Nashville, Tennessee, is piloting the use of the Masimo Radius VSM™ patient-worn vital signs monitor with Masimo Patient SafetyNet™ supplemental remote monitoring in the Emergency Department (ED) and nontraditional care spaces. Launched as part of a successful pilot program aimed at tackling the ongoing crisis of emergency room congestion, Radius VSM has been used on hallway beds, in the emergency medical service offload area, and on patients in the waiting room who are typically only monitored periodically – thus providing continuous, wireless monitoring for those who may otherwise be left vulnerable to unexpected deterioration.
Radius VSM combines the reliability and accuracy of a bedside monitor with the comfort and freedom of a wearable device. With its implementation alongside Patient SafetyNet, clinicians at VUMC are able to remotely monitor vital signs in real time from centralized view stations, simplifying patient data management, enabling quicker intervention during possible deterioration, and enhancing patient safety—even while a patient is up and moving. The modular, scalable monitoring platform offers a range of physiological measurements, including Masimo SET® pulse oximetry, measure-on-inflation noninvasive blood pressure, continuous temperature, respiration rate, and 3-leadwire electrocardiography (ECG).
By monitoring ED patients with Radius VSM, VUMC is transforming spaces that were traditionally devoid of continuous monitoring into areas of proactive patient care. This level of visibility may help clinicians reduce the use of telemetry, potentially saving time and resources and improving patient throughput and prioritization to other parts of the hospital, such as the general ward or medical and surgical wards. Additionally, Radius VSM’s innovative approach not only enhances the patient and clinician experience but exemplifies how cutting-edge technology can be seamlessly integrated into high-pressure settings like the ED to help streamline continuity of care.
The initial success of VUMC’s pilot program is paving the way for an expanded rollout within the ED designed to elevate care for vulnerable patients. Moreover, the promising results may lead to adoption in other areas of the hospital, such as medical and surgical wards, broadening the impact of Masimo’s innovative technology on patient care throughout VUMC. The program also underscores both Masimo’s and VUMC’s commitment to leveraging technology to rethink and improve patient care pathways, setting a new standard for how hospitals manage patient surges in the ED and beyond.
“Rising patient acuity and volume at VUMC necessitate strategic initiatives to augment our care infrastructure,” said Neal Patel, MD, MPH, Professor of Clinical Pediatrics and Chief Informatics Officer for HealthIT at VUMC. “Wireless physiologic monitoring in the ED enhances surveillance and vigilance of each patient’s status even when they are in the waiting room.”
Bilal Muhsin, Chief Operating Officer of Masimo, said, “We are excited to partner with Vanderbilt University Medical Center to bring Radius VSM to vulnerable patients in the emergency department, where continuous monitoring is not the norm. A core tenet of our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications—and one of the ways we achieve that is through continued innovation. With Radius VSM, clinicians have the power of cutting-edge technology in a modular, scalable design that’s both easy to use and comfortable for the patient. The applications are virtually limitless, and I cannot wait to see how the use of this technology is expanded to enhance patient safety not only in the emergency department, but across the continuum of care.”
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-5 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at all 10 top U.S. hospitals as ranked in the 2024 Newsweek World’s Best Hospitals listing.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/.
RPVi has not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2021; 17(8):557-561.
- Estimate: Masimo data on file.
- As ranked in the 2024 Newsweek World’s Best Hospitals listing, available here.
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Radius VSM™ and Masimo Patient SafetyNet™, as well as VUMC’s integration of such technologies in the ED and nontraditional care spaces. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Radius VSM and Masimo Patient SafetyNet, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to our prediction that the initial success of VUMC’s pilot program will result in an expanded rollout within the ED and may lead to adoption in other areas of the hospital, broadening the impact of Masimo technology; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: elamb@masimo.com

Masimo Partners with Qualcomm to Develop Next-Generation Smartwatch Reference Platform for Original Equipment Manufacturers
Comprehensive Platform Combines Masimo’s Industry-leading Biosensing Capabilities with Snapdragon Wearable Platforms to Expand the Wear OS by Google™ Ecosystem
Masimo (NASDAQ: MASI) a leading global medical innovator, and Qualcomm Technologies, Inc., whose Snapdragon® branded platforms power extraordinary consumer experiences, today announced that Masimo is partnering with Qualcomm Technologies to develop a next-generation smartwatch reference platform for original equipment manufacturers (OEMs) building Wear OS by Google smartwatches. The powerhouse combination of Masimo’s clinically proven, breakthrough biosensing technologies – based on its decades of expertise designing industry-leading hospital monitoring solutions – and best-in-class Snapdragon wearable platforms will help scale the wearable ecosystem. Forging the future of wearable devices, the reference platform will allow OEMs to more efficiently build and bring high-performing, premium smartwatches to market. The platform will also benefit from a robust suite of Masimo health and wellness tracking tools that consumers can trust to provide accurate, reliable data; it will use exclusively high-performance and ultra-low power system-on-a-chips (SoCs) alongside industry-leading wireless and cellular communications from Qualcomm Technologies to enable a superior connectivity experience.
Joe Kiani, Founder and CEO of Masimo, said, “We’re thrilled to be able to partner with Qualcomm Technologies, to whom we naturally turned to supply the chips in our own forthcoming Masimo Freedom™ wearable, to craft a truly remarkable reference platform. It’s a platform driven by the belief that when key smartwatch components are supplied by the leading experts in their field, the harmonious whole produces something even greater than the sum of those excellent parts. In short, Qualcomm Technologies and Masimo engineers are working together to optimize the ‘guts’ of the smartwatch. Masimo’s ability to craft precision monitoring technologies and advanced signal processing algorithms, combined with meticulously engineered, high-performing, low-power Snapdragon systems, is a compelling foundation. We expect this new wearable platform to supercharge smartwatch OEMs’ abilities to create competitive, desirable smartwatches for consumers everywhere. I look forward to partnering with a company as well known for its commitment to innovation as Masimo.”
Dino Bekis, Vice President and General Manager, Wearables and Mixed Signal Solutions at Qualcomm Technologies, Inc., said, “Beginning with Snapdragon W5+ Gen 1, this collaboration will significantly broaden the range of smartwatch choices for consumers and further enhance the already exceptional Wear OS experience. With these reference designs, OEMs will benefit from robust, production-ready designs, incorporating Masimo’s state-of-the-art biosensing technology and Qualcomm Technologies’ leading Snapdragon wearable platforms. It will ultimately enable them to seamlessly bring their smartwatches to market rapidly and at scale.”
OEMs who adopt the new reference platform will continue to design and produce their new smartwatches’ physical exteriors and have creative control over the appearance of the user interface. The devices’ interiors, including Snapdragon wearable platforms and Masimo biosensors, will be designed, provided, and tested by Masimo to ensure premium performance and an unmatched user experience. Health and wellness capabilities will feature the same biosensing innovations and analytics that power Masimo W1® and forthcoming Masimo Freedom – technology based on Masimo’s clinically proven, industry-leading Signal Extraction Technology® (SET®). The Masimo reference platform will make all of these components, features, and benefits available for Wear OS smartwatch manufacturers in an easy-to-implement, standardized package.
Snapdragon is a trademark or registered trademark of Qualcomm Incorporated. Snapdragon is a product of Qualcomm Technologies, Inc. and/or its subsidiaries.
Wear OS by Google is a trademark of Google LLC.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-5 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at all 10 top U.S. hospitals as ranked in the 2024 Newsweek World’s Best Hospitals listing.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/.
RPVi has not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2021; 17(8):557-561.
- Estimate: Masimo data on file.
- As ranked in the 2024 Newsweek World’s Best Hospitals listing, available here.
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SafetyNet®, Masimo Radius PPG®, and Masimo SET® and the implementation of Masimo SafetyNet in Saint-Denis Hospital Center (the “Implementation”). These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SafetyNet, Masimo Radius PPG, and Masimo SET®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to our belief of the success of the Implementation and that such success could lead to the deployment of Masimo SafetyNet in other areas; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: elamb@masimo.com

Masimo Partners with Google to Develop a Reference Platform That Helps Device Manufacturers Bring High-performing Wear OS Smartwatches to Market
Comprehensive Smartwatch Reference Platform to Include Key Hardware and Software Components, Including Industry-leading Masimo Biosensing Capabilities
Masimo (NASDAQ: MASI) a leading global medical innovator, today announced a partnership with Google to develop a new reference platform for original equipment manufacturers (OEMs) building Wear OS by Google™ smartwatches. By incorporating Masimo’s breakthrough biosensing technologies – based on its decades of expertise developing industry-leading hospital monitoring solutions – and standardizing smartwatch devices using the Masimo reference platform, OEMs will be able to more efficiently build and bring high-performing Wear OS smartwatches to market. The robust reference platform is designed to support the fast-growing Wear OS ecosystem – including with a suite of health and wellness tracking tools that consumers can trust to provide accurate, reliable data, seamless integration with Android™ smartphones, and a high-quality, high-performance experience.
Joe Kiani, Founder and CEO of Masimo, said, “We’re excited to partner with Google to develop a reference platform for Wear OS that leverages our expert engineering capabilities. Our ability to craft next generation biosensing technologies, precision components, and advanced signal processing algorithms, with rigor and discipline honed over decades, is an excellent foundation for creating a world-class smartwatch reference platform. From hardware to software, Masimo engineers are optimizing the ‘guts’ of the smartwatch – and working with Google to offer OEMs an incredible Wear OS smartwatch solution. Much as numerous leading hospital monitor manufacturers incorporate Masimo monitoring technologies to offer hospitals the best possible patient monitoring solutions, we expect this new wearable platform to supercharge smartwatch OEMs’ abilities to create innovative, competitive, and truly compelling Wear OS smartwatches for consumers everywhere.”
Bjorn Kilburn, GM of Wear OS at Google, added, “Building quality smartwatches with premium features that users have come to expect can be time consuming and costly. With Masimo's reference platform, smartwatch makers are able to benefit from state-of-the-art biosensing technology and quickly bring their Wear OS devices to market, at scale. Together, we're promoting innovation across the Wear OS ecosystem that provides end users with high-performing, feature-rich devices to choose from.”
OEMs who adopt the new Masimo platform, which is expected to be compatible with existing Google apps and services created for the Wear OS platform, will continue to design and produce their new smartwatches’ physical exteriors and have creative control over the appearance of the user interface. The devices’ interiors, including optimized hardware and software components, biosensors, and companion Android smartphone app, will be designed, provided, and tested by Masimo – ensuring superior performance and unmatched user experiences. Health and wellness capabilities will feature the same biosensing innovations and analytics that power the Masimo W1® wearable and forthcoming Masimo Freedom™ smartwatch – technology based on Masimo’s clinically proven, industry-leading Signal Extraction Technology® (SET®). The Masimo reference platform will make all of these components, features, and benefits available for smartwatch manufacturers building Wear OS smartwatches in an easy-to-implement, standardized package.
Google, Android and Wear OS by Google are trademarks of Google LLC.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-5 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at all 10 top U.S. hospitals as ranked in the 2024 Newsweek World’s Best Hospitals listing.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2021; 17(8):557-561.
- Estimate: Masimo data on file.
- As ranked in the 2024 Newsweek World’s Best Hospitals listing, available here.
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SafetyNet®, Masimo Radius PPG®, and Masimo SET® and the implementation of Masimo SafetyNet in Saint-Denis Hospital Center (the “Implementation”). These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SafetyNet, Masimo Radius PPG, and Masimo SET®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to our belief of the success of the Implementation and that such success could lead to the deployment of Masimo SafetyNet in other areas; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: elamb@masimo.com

Saint-Denis Hospital Center in France Implements Masimo SafetyNet® Telemonitoring to Facilitate Early Discharge of Premature Newborns from the Hospital
Leading French Healthcare Facility Adopts an Innovative Telemonitoring Platform as Part of a Mobile Neonatology Unit for the Monitoring of Premature Newborns, Serving Both Patients and Caregivers
Masimo (NASDAQ: MASI) today announced that Saint-Denis Hospital Center is adopting the use of the Masimo SafetyNet® cloud-based telemonitoring platform as part of an experimental mobile neonatology unit aimed at facilitating earlier discharge of premature newborns from the ICU to the home. Launched in February, this pioneering project allows vulnerable neonates to return home safely to their families while remaining under the close supervision of the hospital.
Masimo SafetyNet offers a streamlined approach to remote patient management that can be scaled and tailored to each patient’s unique care needs, offering wireless continuous monitoring and spot-check devices, customizable CarePrograms™ with symptom reporting, and a secure in-hospital clinical portal that allows care teams to keep watch over a large volume of patients. Following discharge, families are sent home with an easy-to-use pulse oximetry sensor, Radius PPG®, powered by clinically proven Masimo Signal Extraction Technology® (SET®), and an intuitive smartphone application. The sensor, worn on the foot of neonatal patients, includes a chip that sends health data to the app, where parents can keep an eye on their baby’s condition and communicate with caregivers. From the hospital, caregivers receive that same data as well as notification logs about changes in a patient’s condition—enabling them to prioritize those who may need care escalation.
Staff members at Saint Denis were already familiar with the benefits of home-based care. Dr. Pascal Bolot, head of neonatal intensive care at Saint-Denis, first implemented home visits in partnership with the ARS, a regional health authority, to address the lack of care following hospital discharge and alleviate the distress of the abrupt shift from hospital to home for families. Saint-Denis’ experience providing home visits was a key advantage when it came to implementing remote neonatal monitoring, alongside 11 other French facilities taking part in the trial. With the integration of Masimo SafetyNet, the pilot has expanded its approach to be more comprehensive and innovative – an approach that, according to Dr. Alizée Lori, the pediatrician at Saint-Denis who oversees the mobile unit, “[brings] premature newborns into a secure home environment equivalent to hospital-grade monitoring quality.” The addition of this technology serves as an opportunity to “put the premature newborn back in the center of the family,” added Dr. Bolot.
The mobile neonatology unit benefits caregivers and families alike. As a general rule, neonatology services do not allow premature babies to return home before the end of 36 weeks of corrected age. Masimo SafetyNet may help make it possible to secure an earlier return home, serving as a relay between hospital staff and families and providing comprehensive support for premature babies in their first few weeks of life. The success of this program could lead to the deployment of Masimo SafetyNet in other care areas, particularly in pediatric care, including home patient management of various conditions.
Dr. Lori commented, “We chose this Masimo solution because of its innovative nature and the practical aspect of wireless physiological data monitoring; it’s easy for families to use and gives our medical team easy access to patients’ health data from the hospital. Since Masimo is used in many neonatology departments, including CH Delafontaine, we were confident in the reliability of the data recorded. This solution allows us to adapt care protocols to meet the needs of our vulnerable patients. Additionally, the Masimo team has supported us since the beginning of the project, and is still available on a daily basis.”
The Saint-Denis Hospital Center, made up of the Delafontaine and Casanova sites, is the only public healthcare facility in the Plaine Commune area, serving a population of 435,000 people. The facility offers 839 hospital beds with adult, pediatric and gynecological emergency services, a large consultation platform, and a type 3 perinatal center for monitoring high-risk pregnancies through high-quality maternal and neonatal care for mothers and children.
Joe Kiani, Founder and CEO of Masimo, said, “Our mission from the beginning has been to improve patient outcomes and reduce cost of care by taking noninvasive monitoring to new sites and applications. And it’s incredible to see how far we’ve come. To see parents be able to be with their newborn baby and each other is extremely gratifying, and to help the dedicated team at Saint-Denis provide earlier discharge yet keep the same level of monitoring is something we’re all proud of. Masimo has long fought to protect vulnerable newborns through innovative continuous monitoring, and it is truly rewarding to see how Masimo SafetyNet—using the same technology we’ve developed and perfected over 35 years—is helping Saint-Denis extend comprehensive care to neonatal patients at home. As healthcare becomes more predictive, preventative, and personalized than ever before, we are committed to innovating solutions that put patients at the center of the care ecosystem.”
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-5 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,6 and is the primary pulse oximetry at all 10 top U.S. hospitals as ranked in the 2024 Newsweek World’s Best Hospitals listing.7 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2021; 17(8):557-561.
- Estimate: Masimo data on file.
- As ranked in the 2024 Newsweek World’s Best Hospitals listing, available here.
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SafetyNet®, Masimo Radius PPG®, and Masimo SET® and the implementation of Masimo SafetyNet in Saint-Denis Hospital Center (the “Implementation”). These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SafetyNet, Masimo Radius PPG, and Masimo SET®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to our belief of the success of the Implementation and that such success could lead to the deployment of Masimo SafetyNet in other areas; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: elamb@masimo.com

Masimo Partners with March of Dimes to Help Parents with Babies in the NICU Make the Transition from Hospital to Home
Company to Donate $100,000 of Stork™ Smart Home Baby Monitoring Systems and Take Part in a March of Dimes Donation Matching Program
Masimo (NASDAQ: MASI) a global leader in innovative monitoring technologies used in top hospitals, and March of Dimes, the leading non-profit organization fighting to improve health outcomes for all moms and babies, are launching a partnership to support new parents with babies in the Neonatal Intensive Care Unit (NICU). Between 9 and 13 percent of babies born require a stay in the NICU due to complex medical needs,1 and many babies born preterm, with birth defects, or with severe health complications spend their first days in the NICU. Through this partnership, Masimo will support March of Dimes’ NICU Family Support® program, which helps over 50,000 families nationwide as they navigate the NICU experience and the transition from hospital to home.
As part of the partnership, Masimo is committed to donating $100,000 of Masimo Stork™ units, a revolutionary, FDA-cleared smart home baby monitoring system designed to deliver peace of mind, to families with the greatest need for support, through March of Dimes’ NICU Family Support program. The NICU Family Support program offers family education, staff training on family-centered care, and an improved experience for parents with a baby in the NICU across more than 70 U.S. partner hospitals.
“Going home is an exciting and long hoped for milestone for new parents, but it is often one of the most overwhelming times for NICU families. Stork, which is powered by the same Masimo SET® sensor technology that’s been trusted in NICUs for decades, was developed to help parents have more peace of mind by keeping them informed about their baby’s health when caring for them at home,” said Joe Kiani, Founder and CEO of Masimo. “Together with March of Dimes and their premier NICU Family Support program, we aim to improve health outcomes for the youngest and most vulnerable patients and provide additional support to new parents as they bring their little ones home from the hospital.”
Stork leverages the same Masimo SET® pulse oximetry technology developed over 35 years ago and is the only device with over-the-counter FDA clearance for use on neonates the day they come home.* Stork monitors a baby’s oxygen saturation level (SpO2), pulse rate (PR), and skin temperature, and notifies caregivers with visual and audible alarms if a baby’s SpO2 or PR readings fall outside of preset ranges.
“March of Dimes is pleased to announce our partnership with Masimo Stork – a collaboration rooted in our mutual commitment to empowering NICU families through knowledge, compassion, and support,” said Kara Gilardi, Associate Vice President, March of Dimes NICU Family Support. “Stork’s sponsorship will support the evidence-based programs we provide that make a world of difference for families in the NICU and the providers who care for them.”
The partnership will also feature key activation moments throughout 2024 and 2025, including NICU Awareness Month programs, donation matching events highlighting Masimo's commitment to supporting families of NICU babies, and video releases that honor NICU families and provide vital resources to assist them in their transition home.
For more information, please visit MasimoStork.com/MOD.
*Masimo Stork is 510(k) cleared for OTC use as a wearable device intended for the monitoring of multiple physiological parameters. Masimo Stork is indicated for spot-checking and continuous monitoring of SpO2 and PR during no motion, motion, and low perfusion conditions in infants and neonates who are 0 to 18 months of age and between 6 and 30 lbs. Masimo Stork OTC is also indicated for continuous skin temperature measurements of infants and neonates who are 0 to 18 months of age and between 6 and 30 lbs. Masimo Stork is indicated for use in home environments.
About March of Dimes
March of Dimes leads the fight for the health of all moms and babies. We provide research, education, advocacy, and to give every family the best possible start. Since 1938, we’ve built a successful legacy to support every pregnant person and every family. Visit marchofdimes.org or nacersano.org for more information.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-6 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,7 and is the primary pulse oximetry at all 10 top U.S. hospitals as ranked in the 2024 Newsweek World’s Best Hospitals listing.8 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/.
RPVi has not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- "Born at Just 1 Pound, a Baby Named Battle Is Thriving After 167 Days in the NICU." Available here.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2021; 17(8):557-561.
- Estimate: Masimo data on file.
- As ranked in the 2024 Newsweek World’s Best Hospitals listing, available here.
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Stork™ and Masimo SET®, as well as Masimo’s partnership with March of Dimes (the “Partnership”). These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Stork and Masimo SET®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks that the Partnership fails to support March of Dimes’ NICU Family Support® program or feature key activation moments as mentioned in this press release; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: elamb@masimo.com

Masimo SET® Pulse Oximetry: Superior Performance, Worldwide Presence, Immeasurable Impact
Masimo SET® Is the Primary Pulse Oximetry at All 10 Top U.S. Hospitals, Monitors More Than 200 Million Patients a Year, and Has Been Shown to Have Unrivaled Accuracy and Reliability in More Than 100 Clinical Studies
Masimo (NASDAQ: MASI) Signal Extraction Technology®, or SET®, pulse oximetry – industry-leading, clinically proven, and used by top hospitals everywhere – continues to overcome the limitations of conventional pulse oximetry and offers unrivaled accuracy through ongoing innovation. As of 2024, Masimo’s foundational SET® technology is now the primary pulse oximetry technology at all ten top U.S. hospitals,1 and is used to monitor more than 200 million patients a year around the world.2 With more than 100 studies demonstrating its ability to outperform other pulse oximetry technologies,2 SET® measures with industry-best SpO2 accuracy specifications4 – achieved with Masimo’s flagship RD SET® sensors – through challenging conditions such as patient motion and low perfusion, across all patient populations, and on all skin tones. With decades of expertise, Masimo is now able to make SET® available not only for traditional hospital bedside monitoring, but in tetherless sensors that promote freedom of movement, in wearable devices worn at home, in baby monitors, and in opioid overdose monitoring solutions – among many others.
Superior Performance
Masimo SET® originated in Founder and CEO Joe Kiani’s desire to find a way to monitor the oxygen saturation of those most precious of patients – neonatal infants – accurately and reliably, even though their frequent movement and poor perfusion stymied traditional pulse oximetry. SET® overcame those seemingly insurmountable obstacles, and Masimo’s continued innovation is driving significant accuracy and reliability milestones even today.
- Masimo SET® is accurate during motion. Before Masimo SET® pulse oximetry, false alarm rates were high,5, 6 often due to motion7, 8 and low perfusion.7 With Masimo SET®, the rate of false alarms dropped by more than 80% and yet true alarm detection improved significantly.7, 8
- Masimo SET® is accurate during low perfusion. A recent peer-reviewed study found that for Masimo SET® SpO2, ARMS* accuracy was “1.37% in all subjects with normal perfusion and 1.64% in all subjects with low perfusion.”9
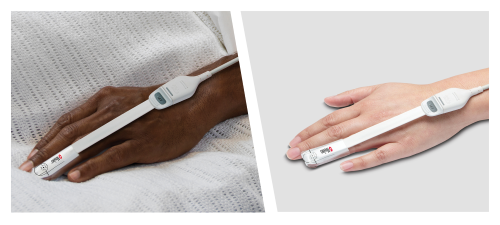
- Masimo SET® is accurate on dark skin. A peer-reviewed publication using appropriately standardized sensor application sites demonstrated that Masimo RD SET sensors had only a 0.15% difference in bias between dark- and light-skinned subjects, which is not clinically significant because pulse oximeters only measure in whole numbers.10 Further analysis showed that RD SET accurately measured SpO2 for both Black and White subjects not only when perfusion was normal but also during low perfusion.9
Improved Outcomes
With its breakthrough performance, Masimo SET® made pulse oximetry a clinically useful tool. Prior to Masimo SET®, not one study had shown that pulse oximetry led to better patient outcomes, despite its ubiquity.11 Masimo SET® has had, and continues to have, a significant impact not only on the lives of innumerable patients around the world, from young to old, as numerous studies have shown, but on clinician workflows, hospital finances, and even the health of our planet.
- Masimo SET® saves lives. A study conducted over 10 years on general floor patients being monitored with Masimo SET® while recovering from surgery found zero preventable deaths or brain damage from opioid-induced respiratory depression.12 Another study has shown that use of SET® led to an 80% reduction in the rate of retinopathy of prematurity (ROP, an eye disorder that can cause blindness in premature babies).13 Of particular note, in multiple studies, involving more than 300,000 newborns, use of SET® as part of screening for critical congenital heart disease (CCHD) has been shown to significantly improve screening sensitivity.14-23
- Masimo SET® saves time. Studies have found that SET® generates 86% fewer false alarms than competitor technologies8 and that use of SET® to monitor general floor patients led to an approximately 60% reduction in rapid response team activations – without compromising patient safety.24
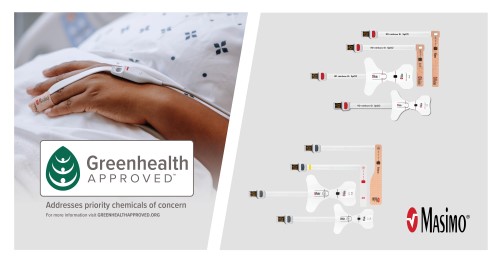
- Masimo SET® saves the environment. Use of Masimo SET® as part of a sensor management strategy that leveraged Masimo’s unique replacement tape offering resulted in a 60% reduction in the volume of single-patient-use sensors.25 And compared to traditional cable-based sensors, Masimo RD SET® sensors produce 84% less waste.26 Earlier this year, Masimo became the first pulse oximetry manufacturer to be recognized with the coveted “Greenhealth approved” sustainability seal of approval by Practice Greenhealth, for RD sensors.
- Masimo SET® saves money. Masimo SET® helped a hospital avoid $7 million in costs over 12 years through the use of a replacement tape sensor management strategy.25 At another facility, use of Masimo SET® to monitor med-surg patients helped clinicians reduce unplanned transfers to the ICU by approximately 50%.24 And, our calculations have shown that use of Masimo SET® and rainbow® technologies could potentially save a 250-bed hospital over $4 million annually.27
“Without SET®, there would be no rainbow® SpHb® or ORi™, no Hospital Automation™ products like Root®, Patient SafetyNet™, or Radius VSM™, and no telemonitoring solutions like Masimo SafetyNet® or Masimo W1®, among so many other achievements,” said Joe Kiani. “Masimo was established precisely to address the limitations of noninvasive monitoring – problems people thought were unsolvable until Masimo’s inventions! We are honored to be the choice of all top ten hospitals in the U.S., but we are far from satisfied. We are committed to continuously building on our legacy of breakthrough innovation and improving the lives of people everywhere.”
Learn more about Masimo SET® here.
*ARMS accuracy is a statistical calculation of the difference between device measurements and reference measurements. Approximately two-thirds of the device measurements fell within +/- ARMS of the reference measurements in a controlled study.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.3 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,13 improve CCHD screening in newborns,14-23 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.12, 24 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,2 and is the primary pulse oximetry at all 10 top U.S. hospitals as ranked in the 2024 Newsweek World’s Best Hospitals listing.1 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at professional.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/.
RPVi has not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- As ranked in the 2024 Newsweek World’s Best Hospitals listing, available here.
- Estimate: Masimo data on file.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Masimo RD SET Sensor Instructions for Use: LAB-10131D, available here.
- Lawless ST. Crying wolf: false alarms in a pediatric intensive care unit. Crit Care Med. 1994 Jun;22(6):981-5.
- Tsien CL, Fackler JC. Poor prognosis for existing monitors in the intensive care unit. Crit Care Med. 1997 Apr;25(4):614-9.
- Shah N et al. J Clin Anesth. 2012 Aug;24(5):385-91.
- Hay W et al. J Perinatol. 2002;360–366.
- Sharma V, Barker S, Sorci R, Park L, Wilson W. Racial effects on Masimo pulse oximetry: impact of low perfusion index. J Clin Monit Comput. 19 Jan 2024.
- Barker SJ, Wilson WC. Racial effects on Masimo pulse oximetry: a laboratory study. J Clin Monit Comput. 2023 Apr;37(2):567-574.
- de-Wahl Granelli A et al. Screening for duct-dependent congenital heart disease with pulse oximetry: A critical evaluation of strategies to maximize sensitivity. Acta Paediatri. 2005;94:1590-1596.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2021; 17(8):557-561.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Slitine N, et al. Pulse Oximetry and Congenital Heart Disease Screening: Results of the First Pilot Study in Morocco. Int J Neonatal Screen 6(53). 30 June 2020.
- Zhao et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. Lancet. 2014 Aug 30;384(9945):747-54.
- Ewer AK et al. Pulse Oximetry Screening for Congenital Heart Defects in Newborn Infants (Pulseox): A Test Accuracy Study. Lancet. 2011 Aug 27;378(9793):785-94.
- de-Wahl Granelli A et al. Noninvasive Peripheral Perfusion Index as a Possible Tool for Screening for Critical Left Heart Obstruction. Acta Paediatr 2007; 96(10): 1455-9.
- Meberg A et al. First Day of Life Pulse Oximetry Screening to Detect Congenital Heart Defects. J Pediatr 2008; 152:761-5.
- Schena F et al. Perfusion Index and Pulse Oximetry Screening for Congenital Heart Defects. J Pediatr. 2017 Apr;183:74-79.
- Hamilçıkan S, Can E. Critical Congenital Heart Disease Screening With a Pulse Oximetry in Neonates. J Perinat Med. 2018 Feb 23;46(2):203-207.
- Jawin V et al. Beyond Critical Congenital Heart Disease: Newborn Screening Using Pulse Oximetry for Neonatal Sepsis and Respiratory Diseases in a Middle-Income Country. PLoS One. 2015; 10(9): e0137580.
- Gunaratne CR, Hewage I, Fonseka A, Thennakoon S. Comparison of pulse oximetry screening versus routine clinical examination in detecting critical congenital heart disease in newborns. Sri Lanka J Child Health, 2021; 50(1): 04-11.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- Palmer, A. Biomed Instrum Technol. 2021. 55(2):59-62.
- Masimo data on file.
- Estimate: Masimo data on file.
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo W1® and Masimo SafetyNet®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo W1 and Masimo SafetyNet, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: elamb@masimo.com

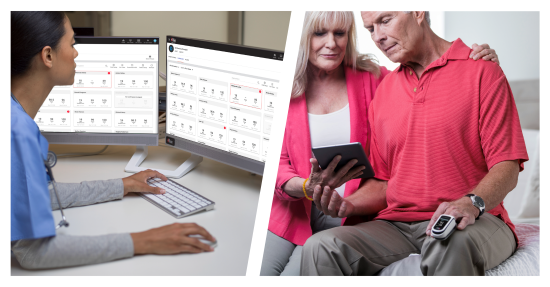
Masimo W1® Medical Watch Receives FDA 510(k) Clearance for Connectivity to the Masimo SafetyNet® Telemonitoring System
Secure Bluetooth® Connectivity Allows Continuous, Accurate Wrist-based Measurements to be Seamlessly Relayed to Caregivers via the Masimo Secure Health Data Cloud
Masimo (NASDAQ: MASI) today announced that the Masimo W1® medical watch has received FDA 510(k) clearance for connectivity, allowing it to be integrated with the Masimo SafetyNet® comprehensive telemonitoring solution. Masimo W1 Medical received FDA clearance last year as the first medical watch to provide continuous oxygen saturation (SpO2) and pulse rate (PR) for over-the-counter and prescription use at home and in hospitals. The combination of Masimo W1 Medical and Masimo SafetyNet allows accurate and reliable patient data, collected conveniently and comfortably via the wrist, to be made available on the Masimo SafetyNet smartphone app and reviewed by remote caregivers, including by hospital clinicians on the web-based Masimo SafetyNet clinician portal – anywhere and at any time.
Joe Kiani, Founder and CEO of Masimo, said, “This is an important milestone for better health outcomes. For the first time caregivers can get customized notifications, based on data from an unobtrusive pulse oximeter worn on the wrist of a patient or loved one, in situations where it’s logistically difficult to provide care in person. Masimo W1 Medical with Masimo SafetyNet opens up a world of possibilities for caregivers looking to improve quality of care and ultimately improve outcomes for those they care for.”
Mr. Kiani continued, “We’ve been excited to see how institutions in Europe and the Middle East are already integrating Masimo W1 with Masimo SafetyNet into their practices in a variety of innovative ways, such as programs that support more confident patient discharge, help anesthesiologists better understand each patient’s unique physiology prior to surgery, and drive toward more predictive, rather than reactive, models of care. And now, with this FDA clearance, we can not only bring these capabilities to the U.S., but we can allow regular people to take better care of each other.”
The clinical power of Masimo W1 Medical comes from its integrated Masimo MW-1 sensor, hardware, and software module, which incorporates over 30 years of Signal Extraction Technology® pulse oximetry and rainbow® Pulse CO-Oximetry knowledge into a single wearable module. It has an integrated optical sensor and electrocardiogram (ECG) electrode pads that can be used to detect physiological signals. The Masimo MW-1 module also processes these signals using Masimo’s proprietary signal processing algorithms to output high-resolution SpO2, PR, perfusion index (Pi), and HR from an ECG.
The continuous data from the Masimo MW-1 module is displayed in real time on the Masimo W1 watch touchscreen in an easy-to-interpret format. With the addition of Bluetooth connectivity, patients can now also take full advantage of the powerful Masimo SafetyNet app, using their phone to input additional symptoms into CarePrograms designed by their clinicians, view their live data and trends, conduct virtual visits with clinicians, and view educational resources.
In turn, clinicians back at the hospital or doctor’s office can examine data and trends and receive customized notifications within the Masimo SafetyNet clinician portal – enabling care providers to manage their patients beyond the boundaries of the hospital. With Masimo SafetyNet, clinicians gain valuable insight into how their patients’ physiological data changes as they go about their day, helping them better understand their patients so that they can identify and prioritize patients who need to follow up. They can more easily track patient progress, tailor remote care programs to match each patient’s needs, and streamline their workflows with high-fidelity reports, rich analytics, and the integration of reliable medical data into electronic medical records (EMRs).
In addition to making patient data available to hospital clinicians via the clinician portal, patients can configure Masimo SafetyNet so that their data can be shared with other types of caregivers, such as a family member taking care of their elderly parents or a loved one. Remote caregivers can also receive customized updates – helping better assess when to check in with their loved one.
Making the secure transmission and storage of patient medical data possible for Masimo SafetyNet is the Masimo SafetyNet cloud: fast, scalable, and reliably available health cloud storage tailored to meet hospitals’ strict standards. The Masimo SafetyNet cloud not only provides the highest level of end-to-end encryption to ensure that data privacy and security are maintained, but gives clinicians access to trended patient data and waveforms wherever they are – supporting more informed clinical insight and analysis. In addition, the comprehensive data set stored in the cloud can be used to generate robust, highly detailed reports, making Masimo SafetyNet a true population health management platform.
Masimo SafetyNet – which also connects to other wearables, like Masimo Radius PPG® for tetherless fingertip pulse oximetry and Radius Tº® for continuous wireless thermometry, as well as a variety of spot-check devices – is now an even more robust, powerful, and comprehensive telemonitoring solution.
The Masimo W1 medical watch and the integrated Masimo MW-1 module are indicated for adults in hospitals, clinics, long-term care facilities, and homes.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at all 10 top U.S. hospitals as ranked in the 2024 Newsweek World’s Best Hospitals listing.9 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/.
RPVi has not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- https://www.newsweek.com/rankings/worlds-best-hospitals-2024/united-states
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo W1® and Masimo SafetyNet®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo W1 and Masimo SafetyNet, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: elamb@masimo.com

New Study Finds That Remote Patient Monitoring with Masimo SafetyNet® Significantly Reduced Rates of Hospital Readmission and ED Visits for Patients Recuperating at Home After Joint Replacement Surgery
Masimo (NASDAQ: MASI) today announced the findings of a prospective study published in the Journal of Orthopaedics in which Dr. Michael DeRogatis and colleagues at St. Luke’s University Health Network (SLUHN) in Pennsylvania, joined by researchers at several additional institutions, evaluated the impact of remote patient monitoring (RPM) using Masimo SafetyNet® and a hospital’s virtual response center (VRC) on 30-day readmission rates for patients undergoing acute postoperative recovery after total joint arthroplasty (TJA). Masimo SafetyNet, a remote patient management and telehealth platform, pairs with a variety of Masimo and third-party devices to seamlessly transmit home-based patient data to hospital clinicians. The researchers found that patients who were remotely monitored after being discharged had significantly lower rates of hospital readmission and ED visits. They concluded, “Remote home monitoring with a virtual response team after outpatient TJA is a feasible way to mitigate readmissions in the acute postoperative period and increase patient satisfaction.”1
Noting that while the trend toward performing TJA as outpatient surgery reduces hospital length of stay (LOS), the practice decreases “available time to monitor for postoperative complications,”1 the authors sought to investigate whether equipping patients with RPM could offer the best of both worlds: outpatient surgery with the ability to track vital signs and more easily stay in touch with patients while they recuperate at home. They enrolled 100 patients who were scheduled to undergo total knee or total hip surgery at SLUHN, divided into two groups: 50 who did not receive RPM after discharge (2021-2022), and 50 who did (2022-2023). Various characteristics (demographics such as age, gender, race, BMI, and marital status; hospital LOS; ASA score; and Charlson Comorbidity Index) were compared between the cohorts and there were no significant differences (p > 0.05), although hospital LOS was slightly shorter in the RPM group (29.4 hours vs. 30.4 hours).
Patients in the RPM cohort were discharged with a Masimo MightySat® pulse oximeter and an Omron blood pressure monitor, provided by Masimo, which connected wirelessly to the Masimo SafetyNet app. For 48 hours after discharge, patients checked their vital signs four times daily. Data recorded by Masimo SafetyNet (including manually entered temperature data) were automatically uploaded to the hospital’s virtual response center for review by hospital clinicians, who reached out to patients as needed to address abnormalities or signs of physiological decline, provide guidance, and, if warranted, recommend returning to the facility for in-person care. Patients in the control group, who did not receive RPM, were discharged with routine TJA postoperative instructions.
The researchers found that in the RPM cohort, 10 patients (20%) recorded abnormal vital signs and 2 patients (4%) visited the ED; no patients were readmitted to the hospital. In the control cohort, 6 patients (12%) visited the ED and all visits resulted in hospital admission – significantly higher rates of ED visitation and hospital readmission (p = 0.03). The causes for the readmissions included two hip dislocations, cellulitis (soft tissue infection), and uncontrolled pain. The authors noted that other than the dislocations, these readmissions “could have been prevented with remote home monitoring.”1 They conservatively estimated a readmission cost of $7,000 per patient.
Although they did not conduct a formal cost analysis of the RPM program, the authors estimated that, after taking into account the potential cost avoidance of fewer hospital readmissions, for every 1,000 TJA surgeries performed, the RPM program “could potentially result in a hospital savings of $800,000.”1
The researchers also surveyed patients in the RPM cohort about their experience and found that most held a favorable view of the program: 79% strongly or somewhat agreed that RPM helped in their care at home, 79% strongly or somewhat agreed that it made them feel safer, and 79% strongly or somewhat agreed they would recommend RPM to someone they know.
The authors concluded, “As total joint replacements are increasingly being performed in the outpatient setting, postoperative patient monitoring from home is a feasible way to help mitigate readmissions in the postoperative period. The utility of a virtual response center would be to identify, in real time, certain patient metrics that could indicate potential complications and ultimately allow for more timely intervention that may prevent morbidity and readmissions. Efforts to minimize costs should not be implemented at the expense of patients’ health outcomes with a goal to find an appropriate balance between both agendas.”1
Masimo SafetyNet is not intended to be used for real-time monitoring.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-8 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,9 and is the primary pulse oximetry at all 10 top U.S. hospitals as ranked in the 2024 Newsweek World’s Best Hospitals listing.10 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/.
RPVi has not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- DeRogatis MJ, Pellegrino AN, Wang N, Higgins M, Dubin J, Issack P, Sokunbi G, Brogle P, Konopitski A. Enhancing recovery and reducing readmissions: The impact of remote monitoring on acute postoperative care in outpatient total joint arthroplasty. J Ortho. 26 June 2024. 58(2024). DOI: 10.1016/j.jor.2024.60.028.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- https://www.newsweek.com/rankings/worlds-best-hospitals-2024/united-states
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SafetyNet® and MightySat®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SafetyNet and MightySat, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks that the researchers’ conclusions and findings may be inaccurate; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: elamb@masimo.com

Masimo Announces Sleep Halo™, Advanced Sleep Analysis for the Masimo W1®
Sleep Halo Sleep Metrics Are Based on Masimo W1’s Continuous Pulse Oximetry Measurements and Advanced Halo AI Technology
Masimo (NASDAQ: MASI), today announced that the Masimo W1® Sport advanced health tracking wearable is gaining a powerful new feature: scientifically based sleep analysis with Sleep Halo™. Sleep Halo offers overnight sleep data tracking with an unmatched 70,000+ daily measurements of second-by-second continuous health data.
Sleep Halo uses continuous Masimo pulse oximetry and machine learning to provide meaningful insights into the quality of an individual’s sleep, including overall timing, duration of sleep stages, periods of rest and wakefulness, episodes of desaturation, and more. The nightly analysis, including a Sleep Halo score – an algorithmic calculation representing your overall sleep quality – is visualized on the companion Masimo Health smartphone app. The Sleep Halo score and sleep analysis will be featured in all future Masimo wearables, including the upcoming Masimo Freedom™ Watch and Band.
Joe Kiani, Founder and CEO of Masimo, said, “We’re truly excited to launch Sleep Halo for Masimo W1 Sport. For 35 years, Masimo has been dedicated to using breakthrough engineering to help improve lives. Masimo W1 is unique among wearables in being able to continuously measure the wearer’s SpO2 and PR – and that gives us an opportunity to provide a truly informed, robust analysis of how one sleeps. As always, we are committed to providing scientifically backed measurements, which are based on our unmatched, accurate technology, not ‘tarot card logic’ novelties. We benchmarked Sleep Halo with EEG to confirm its stages of sleep are comparable to sleep analysis. We hope that with this new scientifically based Sleep Halo, our customers can improve their sleep hygiene, which has been shown to improve life.”
Today’s consumers are limited to choosing from among a variety of consumer wearables, which use intermittent and often inaccurate data, heavily reliant on movement – which cannot provide reliable or actionable sleep metrics. Masimo W1 – which has been shown to be significantly more accurate than the leading consumer wearable1 – is designed to provide sophisticated and insightful sleep analysis using technology based on Masimo SET®, the primary pulse oximetry technology at all top 10 U.S. hospitals as ranked in the 2024 Newsweek World’s Best Hospitals listing.2 Rigorously tested against reference laboratory measurements, including EEG data, Sleep Halo makes it possible to bring reliable, insightful sleep analysis to everyday people around the world, on a nightly basis, via a lightweight, comfortable wearable, from the convenience of their own home.
Existing Masimo W1 Sport owners will soon be able to update their watch firmware and Masimo Health app to begin their sleep tracking journey. Masimo W1 is available for purchase at www.Masimo.com.
Also available is Masimo W1 Medical, the first and only FDA-cleared watch to provide continuous, real-time SpO2 and PR with an indicator when measurements are outside their normal ranges.
Masimo W1 Sport and Sleep Halo are for general health and wellness purposes.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.3 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,4 improve CCHD screening in newborns,5 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.6-9 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,10 and is the primary pulse oximetry at all 10 top U.S. hospitals as ranked in the 2024 Newsweek World’s Best Hospitals listing.2 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/.
RPVi has not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Masimo internal whitepaper on file.
- https://www.newsweek.com/rankings/worlds-best-hospitals-2024/united-states
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo W1® and Sleep Halo™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo W1 and Sleep Halo, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to the assumption that Sleep Halo will be available on future Masimo wearables including Freedom™ Watch and Band; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: elamb@masimo.com

Masimo and Cleveland Clinic Collaborate to Improve Hospital Remote Care
Masimo (NASDAQ: MASI), a leading global provider of medical technology and hospital automation solutions, and Cleveland Clinic, a nonprofit academic medical center, announced today the launch of a new partnership centered around hospital-based remote patient monitoring (RPM), including TeleCritical Care. This will include the integration of Cleveland Clinic’s critical care (eHospital) and non-critical care (eCMU) central patient monitoring platforms with the Masimo Hospital Automation™ platform. The goal is to provide tools for clinicians that offer enhanced situational awareness and clinical decision-support for hospitalized patients, including the critically ill.
The collaboration will include joint development initiatives on predictive analytics and AI-based algorithms for improving cardiac care.
Cleveland Clinic’s existing critical care and non-critical care central monitoring platform provides continuous monitoring of a range of vital signs, including ECG, for both ICU and non-ICU patients at more than a 2,000-bed capacity. Its hospital-based RPM programs serve 11 hospitals, providing intensivist monitoring, 24/7 critical care nursing, and patient management. Cleveland Clinic’s programs1 have reduced patient mortality and reduced ICU length of stay while increasing caregiver satisfaction.2 With this partnership, Masimo and Cleveland Clinic hope to bring these innovations and patient benefits to other healthcare systems in the future. The aim of the enhanced program is to increase awareness and facilitate triage with proactive responses to changes in a patient’s condition. This improves patient care and saves lives by ensuring the highest risk patients are identified and receive timely treatment while maintaining quality of care for all patients.1,2
The Masimo Hospital Automation platform offers technologies designed to help clinicians improve patient care at the bedside, across the care continuum. Masimo Hospital Automation includes monitoring and wearable technologies, high-fidelity medical device integration, system-wide applications for surveillance and data visualization, and novel AI capabilities that support intelligent patient prioritization and help clinicians identify changes in patients’ condition more efficiently.
These decision-support tools are integrated into the Masimo Hospital Automation platform and utilize the Halo engine, technology which identifies deterioration patterns in multiple physiological parameters simultaneously, in real time. The Halo tools include Halo ION®, a comprehensive, scalable, and customizable continuous early warning score to help streamline patient assessment and clinical workflows. As part of their work together, Masimo and Cleveland Clinic are partnering to jointly develop an additional Halo-based decision-support tool to support clinicians with earlier detection of adverse events – ultimately helping them manage and improve patient outcomes more effectively, for low-, mid-, and high-acuity patients.
“We see great opportunities to enhance remote care, particularly for critically ill individuals,” said Chiedozie Udeh, M.D., Medical Director of ICU Operations at Cleveland Clinic. “By combining our technical and clinical expertise, we aim to improve situational awareness for clinicians and continue to improve outcomes for patients.”
Thomas Callahan, M.D., Staff Cardiologist at Cleveland Clinic’s Heart, Vascular & Thoracic Institute, and principal investigator for the AI study, added, “We look forward to exploring the effects of next-gen inpatient wearables, and as the capabilities of AI continue to advance, studying the potential impact on the care of cardiac patients, including those undergoing cardiac surgery.”
Joe Kiani, Founder and CEO of Masimo, said, “We are truly honored to have the opportunity to partner with Cleveland Clinic to advance patient care. By harnessing Masimo’s AI-powered decision support tools, automation solutions, and monitoring devices, alongside Cleveland Clinic’s vast clinical expertise and dedication to providing the highest quality, most innovative care, our partnership has the potential to significantly ease staff shortages, better standardize care, and promote intensivist- and specialist-led care. Ultimately, we will make significant strides in shifting from reactive to predictive and proactive care – improving patient outcomes, safety, and quality of care across the board.”
Dr. Chiedozie Udeh has a conflict of interest with Cleveland Clinic’s eHospital platform and may benefit from commercialization of eHospital technology developed by the Company. These financial interests are being managed by Cleveland Clinic and are within permissible limits established by the Institutional Conflicts of Interest Policy. eHospital is not a Masimo product.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.3 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,4 improve CCHD screening in newborns,5 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.6-9 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,10 and is the primary pulse oximetry at all 10 top U.S. hospitals as ranked in the 2024 Newsweek World’s Best Hospitals listing.11 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/.
RPVi has not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Cantillon et al. JAMA 15-10101. Issued August 2016.
- Udeh C, Canfield C, Brown A et al. ICU Telemedicine and Clinical Factors Related to 30-Day Mortality: A Retrospective Cohort Study, Critical Care Medicine. January 2021 - Volume 49 - Issue 1 - p 9 doi: 10.1097/01.ccm.0000726092.34718.60.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- https://www.newsweek.com/rankings/worlds-best-hospitals-2024/united-states
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Hospital Automation™, Halo, and Halo ION®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Hospital Automation, Halo, and Halo ION, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks that Masimo may not realize the expected benefits and goals that may be achieved by its collaboration with Cleveland Clinic; risks that Masimo and Cleveland Clinic fail to collaborate in the areas stated in this press release as planned; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: elamb@masimo.com
Cleveland Clinic
Andrea Pacetti
Phone: (216) 316-3040
Email: Pacetta@ccf.org
Alicia Reale-Cooney
Phone: (216) 408-7444
Email: Realeca@ccf.org

Masimo Joins Forces with the Mobilize Recovery 2024 Bus Tour
Collaborative Partnership Designed to Enhance Nationwide Efforts to Combat the Opioid Epidemic and Improve Addiction Recovery
Masimo (NASDAQ: MASI), a leading global medical technology innovator, today announced that it is partnering with Mobilize Recovery, a nonprofit organization dedicated to ending America’s addiction and overdose crisis, by sponsoring and joining the Mobilize Recovery 2024 bus tour. The tour, which kicks off on September 20th in Los Angeles, with the first stop in Irvine, at the Masimo headquarters, is set to travel to 15 key cities around the U.S. to raise awareness, educate, and support communities around the country as they seek to prevent overdose and make sustainable improvements in addiction recovery outcomes. As part of this sixth annual initiative, Mobilize Recovery and Masimo will be showcasing two key Masimo innovations, Opioid Halo™ and Bridge™, which are designed to help people who are using opioids stay safe and reduce withdrawal symptoms during addiction recovery, respectively.
Founded by Mr. Ryan Hampton, Mobilize Recovery brings together recovery advocates, nonprofit organizations, allies, business leaders and innovators like Masimo, government partners, and like-minded community-based organizations with an interest in creating sustainable change and community solutions, as well as celebrating recovery from substance abuse disorder and mental health challenges. Engaging year-round with mobilizers, Mobilize Recovery provides partnership support and education to uplift and sustain partners’ work; help them develop action-oriented, measurable, scalable goals; raise public awareness; and prevent overdose.
Ryan Hampton, Founder and Executive Director of Mobilize Recovery, said, “Mobilize Recovery champions a grassroots approach, empowering advocates, innovators, businesses, and communities battling the overdose crisis firsthand. We see Masimo as a leading innovation partner in the recovery movement, uniquely positioned to drive technological advancements that make a real impact. We're thrilled to collaborate with a company so committed to leveraging technology for groundbreaking solutions in recovery. Masimo's Opioid Halo and Bridge have shown immense promise in helping opioid users, and we're eager to see their full potential realized. In Masimo’s CEO, Joe Kiani, we've found a passionate voice advocating for overcoming this epidemic, a true thought leader in the recovery field with an unwavering dedication to making a difference.”
Joe Kiani, Founder and CEO of Masimo, added, “We are excited to sponsor Mobilize Recovery, partner with their amazing team, and help kick off the bus tour in September. Masimo is committed to creating evidence-based solutions that fill in existing gaps in patient support networks. We were honored to be chosen by the FDA as a winner of their FDA Opioid Innovation Challenge for our solution to help prevent opioid overdose. With Opioid Halo, we became the first winner to have an authorized device to address the crisis. Bridge, in turn, is the first FDA-cleared medical device to help in the reduction of opioid withdrawal symptoms. Together, we believe these products, and the amazing efforts of Mobilize Recovery and its partners, can make a real difference in fighting the opioid crisis – as well as help eliminate the stigma around addiction.”
Opioid Halo, an opioid overdose prevention and alert system, was granted a De Novo by the FDA in 2023, making it the first and only FDA-cleared monitoring solution for detecting opioid-induced respiratory depression (OIRD). Opioid Halo advances the forefront of continuous monitoring through its unique Opioid Halo engine, an advanced pattern recognition algorithm which helps detect and quantify the risk of severe OIRD. Combined with its innovative distributed architecture, Opioid Halo helps to manage and send escalating alarms to family members, friends, and caregivers, notifying them that help may be needed due to an opioid overdose – including triggering an automatic wellness call, which may lead to EMS being dispatched.
Bridge, a drug-free opioid withdrawal device, uses neuromodulation to aid in the reduction of symptoms associated with opioid withdrawal. Bridge, which has also been granted a De Novo by the FDA, is the first evidence-based, drug-free, non-surgical device of its kind. While medication-assisted treatment can be effective in helping treat opioid withdrawal symptoms, Bridge may help significantly reduce those symptoms, within 30 minutes, helping users make progress in their treatment by bridging the gap on the road to recovery. Bridge fits behind the ear – applied by a healthcare provider in a short, non-surgical procedure – and works by sending electrical impulses to the nerves around the ear, which transmit them to the brain, providing up to five days of continuous relief from withdrawal symptoms.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.9 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/.
RPVi has not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- As ranked in U.S. News and World Report. health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Opioid Halo™ and Bridge™, as well as the potential benefits of Masimo’s partnership with Mobilize Recovery. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Opioid Halo and Bridge, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: elamb@masimo.com

Germans Trias i Pujol Hospital in Spain Launches Telehealth and Remote Patient Management Program Using Masimo W1® Medical Watches and Radius VSM™ Wearable Continuous Vital Signs Monitors
Scope of the SESHAT Project at Pioneering Hospital, Part of the Catalan Health Institute, Includes Deployment of up to 1,000 Masimo W1 Watches, 100 Radius VSMs, and Numerous Additional Masimo Hospital Automation™ Solutions
Masimo (NASDAQ: MASI) today announced that Germans Trias i Pujol, a pioneering hospital serving more than 800,000 people in north Barcelona, Spain, is launching a breakthrough telehealth and remote patient management initiative using Masimo technology. The SESHAT project is focused on using advanced wearable technologies and wireless connectivity to allow remote clinicians to keep track of patients’ physiological data in near real time wherever they are, whether the patient is in another part of the hospital or at home. The project, which began in the fourth quarter of 2023 and is slated to last at least three years, includes the implementation of up to 1,000 Masimo W1® medical watches, 100 Radius VSM™ Wearable Continuous Vital Signs Monitors, 10 Patient SafetyNet™ Systems, and a host of additional Masimo Hospital Automation™ products.
Joe Kiani, Founder and CEO of Masimo, said, “We are honored to partner with Germans Trias i Pujol to support the SESHAT project. When I founded Masimo 35 years ago, our mission was to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. Now, with decades of innovation and refinement, we are bringing our pioneering accurate and continuous monitoring technologies to even more new sites and applications, including the home – with powerful wearable, tetherless, and remote modalities like Masimo SET®-based Masimo W1 and Radius VSM. Our breakthrough predictive algorithm Halo™, AI-powered tools, and connectivity and automation solutions in turn make that accurate, precise, rich stream of actionable patient data available to clinicians when and where they need it, helping patients feel connected and cared for, and informing evermore insightful decisions about care. Ultimately, we believe this will improve outcomes while reducing the cost of care, in keeping with our founding mission. We look forward to gleaning innumerable insights and improving 800,000 lives with the forward-thinking clinicians of Germans Trias i Pujol.”
Dr. Oriol Estrada, Director of Healthcare Strategy and Innovation at Germans Trias, commented, “Project SESHAT, named for the Egyptian goddess of writing and measurement, aims to transform healthcare from a reactive to a predictive model thanks to continuous and real-time reliable patient data measured through wearable technology, both in hospital and at home.”
The SESHAT project arose from needs identified during the COVID pandemic and centers on the remote monitoring of patients with complex care requirements. The project's goal is to obtain real-time physiological data from patients and have that data help clinicians improve health outcomes. By facilitating better tracking of each patient’s physiological changes, and combining such changes with other available health data, SESHAT project clinicians hope to design and validate better prospective indicators of clinical progress and therapeutic adherence. Their goal is ultimately to help institutions transition from a reactive care model, in which healthcare teams treat symptomatic diseases, to a more proactive model in which teams receive information that enables them to anticipate complications or select more suitable medications to ensure a better therapeutic response, based on each patient's profile.
“To achieve these objectives,” continued Dr. Estrada, “wearable technology that is robust, reliable, and does not hinder the patient's normal life had to be selected. After analyzing various options, Masimo technology embedded in medical-grade wearable devices such as Masimo W1 and Radius VSM was chosen. A decisive factor in this choice was Masimo's willingness to become a technological partner beyond just being a technology provider. We have established a strategic collaboration whereby the SESHAT project will serve as a validation field for technological improvements and identification of new needs in which both institutions will cooperate. When deciding, it was important for the project leaders to work with a leading company in the monitoring technology sector that offers a portfolio with a global solution for all phases of patient care: from the hospital to the home, while also providing integration with Hospital Information Systems.”
The Germans Trias i Pujol University Hospital of Badalona belongs to the Catalan Health Institute. It is a high-tech center that provides health services to a population of 800,000 inhabitants in the North Metropolitan area of Barcelona. Since its foundation, the hospital has been a pioneer in the development and care in various areas of excellence, such as infectious, cardiovascular, inflammatory, oncological, and respiratory diseases, among others. It encompasses a powerful biomedical research campus with 12 internationally renowned institutions, employing over 6,000 professionals and researchers in the field of health. Clinical innovation—transforming clinical problems into opportunities for improving outcomes—is one of the institution's strategic pillars.
Halo is not FDA cleared and is not available in the U.S.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.9 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/.
RPVi has not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- As ranked in U.S. News and World Report. health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others,vstatements regarding the potential effectiveness of Masimo W1™, Radius VSM™, Hospital Automation™, SET®, the partnership of Masimo and Germans Trias i Pujol Hospital (the “Partnership”), and the prospect of SESHAT project. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo W1, Radius VSM, Hospital Automation, and SET®, as well as the Partnership and the SESHAT project ,contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to the assumption that the SESHAT project will last at least three years; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: elamb@masimo.com

Masimo Receives Sustainability Seal of Approval from Practice Greenhealth
Masimo Becomes First Pulse Oximetry Manufacturer to Be Recognized with Coveted “Greenhealth Approved” Seal
Masimo (NASDAQ: MASI), a leading medical technology innovator, and Practice Greenhealth, a prominent organization dedicated to advancing sustainability solutions for healthcare providers, today announced that Masimo is now a recipient of the Greenhealth Approved seal. Masimo’s products – in particular its line of RD pulse oximetry sensors – are the first pulse oximetry products to receive the Greenhealth sustainability seal. The seal is only granted to products that meet specific sustainability criteria, making it a reliable indicator for health care providers seeking to identify environmentally preferable products without the time and expense needed to conduct their own independent verification.
Bilal Muhsin, Chief Operating Officer, Masimo, said, “Masimo is committed to both excellent patient care and environmental responsibility. The RD pulse oximetry sensor line was designed with the environment in mind, so we are pleased and proud to be the first to receive the Greenhealth Approved seal in this category.”
Masimo continually strives to make its products, packaging, and facilities more environmentally friendly and has implemented a variety of green initiatives, including designing products that promote sustainability by eliminating unnecessary material from being introduced into the product stream, expansion of its sensor recycling program, which is committed to achieving “zero waste to landfill,” and partnership with sustainability-focused organizations such as Practice Greenhealth.
From sensor design to disposal, Masimo uses materials that are less wasteful without compromising quality or performance. In addition to meeting the chemicals criteria of Health Care Without Harm, RD single-patient-use pulse oximetry sensors also:
- Reduce waste by 84% when compared to cable-based sensors1
- Achieve zero waste to landfill through Masimo’s sensor recycling program
- Allow for “reprocessing at the bedside” with replacement tapes
- Offer best-in-class SpO2 accuracy specifications of 1.5% ARMS* and accurately measure on patients with varying skin pigmentation and low perfusion2,3
- Maximize patient comfort with low-profile, lightweight components
- Use the same, standardized connector for multiple sensor technologies (e.g. SET® and rainbow®)
The review process for the Greenhealth Approved seal includes the submission of a complete ingredient list along with proof of certifications for the category’s criteria. “Greenhealth Approved removes obstacles that previously made purchasing sustainable products difficult,” said Paul Bogart, Executive Director of Practice Greenhealth. “Normally each health care provider conducts its own product review, often without extensive experience in chemistry and sustainability. We are helping streamline and centralize this approach, working closely with each supplier and completing a detailed product review using trusted criteria. Greenhealth Approved allows health care providers to quickly and easily identify and purchase sustainable products that decrease their impact on the environment.”
Providers interested in finding out which products carry the Greenhealth Approved seal can check the website and sign up for regular updates as products are approved for the seal. Practice Greenhealth is the leading sustainable health care organization, delivering environmental solutions to more than 1,700 hospitals and health systems in the United States and Canada.
Masimo will be exhibiting its Greenhealth-approved RD pulse oximetry sensors at CleanMed 2024, the premier health care sustainability conference, hosted by Practice Greenhealth and Health Care Without Harm, on May 21st - 23rd in Salt Lake City, Utah.
*ARMS accuracy is a statistical calculation of the difference between device measurements and reference measurements. Approximately two-thirds of the device measurements fell within ± ARMS of the reference measurements in a controlled study.
About Practice Greenhealth
Practice Greenhealth is the leading sustainable health care organization, delivering environmental solutions to more than 1,700 hospitals and health systems in the United States and Canada. Partnership opportunities include NGOs, nonprofits, government, academic institutions, and the health care value chain.
Practice Greenhealth is the innovation hub scaling the work of Health Care Without Harm, an organization that seeks to transform health care worldwide. For more information, visit practicegreenhealth.org.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.4 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,5 improve CCHD screening in newborns,6 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.7-10 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,11 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.12 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/.
RPVi has not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Internal data on file.
- Barker SJ, Wilson WC. J Clin Monit Comput. 2023;37:567-574.
- Sharma, et al. J Clin Monit Comput. 2024 Jan;38:347-354.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- As ranked in U.S. News and World Report. health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others,statements regarding the potential effectiveness of Masimo RD pulse oximetry sensors and the benefits for them to be Greenhealth Approved. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results;risks related to our belief that Masimo's unique technologies, including Masimo RD pulse oximetry sensors, contribute to positive clinical outcomes and patient safety; risks that Masimo fails to exhibit its Greenhealth-approved RD pulse oximetry sensors at CleanMed 2024; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: elamb@masimo.com
Practice Greenhealth
Ella Schwotzer
Phone: (866) 310-3320
Email: press@practicegreenhealth.org

Masimo and Denon Introduce White PerL™ and PerL Pro™ Earbuds, Redefining Style and Customization in Personalized Audio
The only earbuds on the market that analyze your hearing profile to generate truly custom sound made just for you
Masimo (NASDAQ: MASI), a global leader in innovative noninvasive monitoring technologies, and Denon, a pioneer in audio technology with over a century of audio expertise, proudly unveil the latest evolution of its acclaimed Denon PerL™ and Denon PerL Pro™ True Wireless earbud lineup with the introduction of White PerL. Denon PerL represents the epitome of personalized audio listening.
The two Denon PerL models are engineered with Masimo’s proprietary Adaptive Acoustic Technology™ (AAT™) to deliver the only earbud that puts hearing personalization at the forefront of the listening experience. Masimo AAT works by detecting an individual’s inner ear response from carefully curated sound frequencies sent into the ear canal and then creating a custom hearing profile based on each individual’s hearing sensitivities – helping to enhance sound depth, detail, and clarity for a superior listening experience.
Joe Kiani, Founder and CEO of Masimo, stated, “PerL continues to propel us into a new era of personalized listening, and Masimo is proud to lead the charge. With our Adaptive Acoustic Technology integrated into Denon's PerL earbuds, we're not just changing the way we listen to music; we're revolutionizing the future where every individual can enjoy sound tailored precisely to their unique hearing profile, setting a new standard for immersive audio."
Masimo's AAT has raised the bar to new heights for personalized audio. By harnessing Masimo's expertise, Denon PerL goes beyond conventional audio tuning, ensuring that each user's unique hearing profile is fully understood.
Your Music Tuned Your Way
From person to person, hearing differences impact the listening experience. Denon PerL with Masimo AAT automatically tunes the sound based upon each individual’s inner ear response to enhance sound depth, details, and clarity for a supreme personalized listening experience. The Denon PerL Headphone app for iOS and Android simplifies the creation of your personalized hearing profile through a step-by-step adjustment process. The app also allows you to adjust listening settings and change your bass and treble equalization preferences while still using your optimized hearing profile.
Elevating the Audio Experience with Denon's Legacy of Excellence
Drawing from over a century of audio mastery, Denon ensures that both PerL models embody the brand's signature sound superiority. By combining the brand’s renowned signature spacious and vivid sound with Masimo's cutting-edge technology, Denon PerL offers unmatched depth, detail, and clarity every session.
Unparalleled Versatility and Functionality
In addition to personalized sound, the Denon PerL earbuds boast an array of features designed to adapt to the user's lifestyle seamlessly. One of the problems with many earbuds is that they easily fall off the ear. This problem has been substantially reduced due to Denon PerL’s unique mechanical design.
Whether immersing oneself in music with active noise cancellation or staying connected to the surroundings with social mode, Denon PerL has features to accommodate a variety of lifestyles. Enjoy advanced functions with Denon PerL Pro such as spatial audio, which creates a more immersive surround sound atmosphere in a compact and comfortable format, and adaptive active noise cancellation that dynamically adjusts to each user's environment.
Discover the power of truly personalized listening, as Denon redefines the audio experience. The new color of Denon PerL and Denon PerL Pro is now available at www.Denon.com for $199 and $349, respectively.
About Denon
Denon, a leading manufacturer of premium audio equipment, is dedicated to delivering the ultimate listening experience to music lovers worldwide. Since 1910, Denon has been at the forefront of technological advancements in audio engineering, setting the standard for high-quality sound systems that are built to last and cater to the diverse needs of music enthusiasts, from audiophiles to casual listeners. For more information, visit Denon.com.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.9 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- As ranked in U.S. News and World Report. health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Hospital Stork™ and SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results;risks related to our belief that Masimo's unique technologies, including Stork and SET®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to the assumption that Stork OTC monitoring and alarm features will be available through the Masimo Stork App and Stock OTC bundles will be available online and at retailers this summer; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Matt Whewell
Phone: (720)-838-0691
Email: Matt.whewell@masimo.com

Masimo Receives FDA Clearance for Stork™ Over-the-Counter (OTC) Baby Monitoring System
With This Clearance, Stork Provides Continuous Monitoring of Oxygen Saturation, Pulse Rate, and Skin Temperature, with Alarms, for Babies up to 18 Months, at Home, Without a Prescription
Masimo (NASDAQ: MASI), a global leader in innovative monitoring technologies currently celebrating its 35th anniversary, announced FDA clearance of Stork™, a baby monitoring system that provides alarms to parents or other caregivers, for use with healthy babies 0-18 months of age, without the need for a prescription.
The over-the-counter (OTC) version of Stork utilizes the same Masimo pulse oximetry technology that monitors more than 10 million babies in hospitals every year1 and is used at 9 of the top 10 U.S. hospitals.2 The FDA-cleared OTC version of Masimo Stork monitors a baby’s key vitals data including oxygen saturation level (SpO2), pulse rate (PR), and skin temperature; crucially, Stork notifies caregivers with visual and audible alarms if a baby’s SpO2 or PR readings fall outside of preset ranges.
Stork leverages the same pulse oximetry technology that has been used on babies in the neonatal intensive care unit (NICU) for decades, helping to improve health outcomes for the youngest and most vulnerable patients. Known as Signal Extraction Technology®, or SET®, this technology has helped clinicians reduce the incidence of neonatal blindness from retinopathy of prematurity3 and has led to significant improvements in screening newborns for critical congenital heart disease.4
“Bringing your new baby home from the hospital is exciting, but can be nerve-wracking—and it’s something I remember vividly from when my son was born and required at-home monitoring,” said Joe Kiani, Founder and CEO of Masimo. “I believe that by empowering parents and caregivers with the same Masimo pulse oximetry technology that hospitals have been using on babies for decades, we can help them feel more informed and confident when caring for their baby. It is particularly fitting that we received this clearance and are making this announcement as we celebrate the 35th anniversary of the company I founded with a mission to improve lives everywhere, especially those of the youngest and most fragile among us. Thirty-five years ago I envisioned Stork. I am so proud of our team for Stork – it is a culmination of science and design at its best.”
Stork bundles are currently available at major and specialty retailers nationwide and at masimo.com as a non-medical device for general health and wellness purposes. Alongside the FDA clearance, Masimo will make Stork’s new alarm features available to all existing and new Stork users through the Masimo Stork App, in an update scheduled to be released this summer. FDA-cleared OTC Stork bundles will be available for purchase online and at retailers this summer.
Masimo’s patented SET® sensor technology nests within the Stork boot, which is made from an ultra-soft, medical-grade silicone that conforms gently to the baby’s skin and is available in three sizes to ensure a perfect fit as the child grows. The sensor embedded in the boot is the product of meticulous engineering that harnesses decades of expertise in noninvasive monitoring to detect babies’ SpO2, PR, and skin temperature continuously, with unprecedented accuracy and dependability.
Masimo Stork has a sleek, minimalist design that fits seamlessly into any nursery and on the baby’s foot. The Stork Vitals+ bundle includes the boot with sensor that monitors baby’s skin temperature, PR, and SpO2, and a 2K Quad High-Definition (QHD) capable camera with technology supported by the TODA platform from Like Minded Labs. The camera hardware and software architecture are designed to leverage and be compatible with future edge AI-based features, which are in development. The first application of the edge AI technology will be identifying babies who have turned face down. For those who do not require streaming video, or detection via video of babies being face down, the Stork Vitals bundle replaces the camera with a hub, which connects the Stork vital signs sensor/boot to the Stork app, while still allowing parents to hear their baby. Stork also monitors the baby’s room conditions (ambient temperature and humidity).
Masimo Stork is 510(k) cleared for OTC use as a wearable device intended for the monitoring of multiple physiological parameters. Masimo Stork is indicated for spot-checking and continuous monitoring of SpO2 and PR during no motion, motion, and low perfusion conditions in infants and neonates who are 0 to 18 months of age and between 6 and 30 lbs. Masimo Stork OTC is also indicated for continuous skin temperature measurements of infants and neonates who are 0 to 18 months of age and between 6 and 30 lbs. Masimo Stork is indicated for use in home environments.
Masimo Stork can be used to supplement a caregiver’s decision to seek additional guidance for the care of an infant or neonate. It is not intended to provide notifications for every episode of the unexpected occurrences of elevated or depressed PR or low SpO2; rather, Masimo Stork is intended to provide a notification only when sufficient data are available for analysis.
Masimo Stork is not intended to replace the monitoring, diagnosis, or treatment provided by a physician or healthcare provider. Masimo Stork is not intended for use with infants and neonates previously diagnosed with cardiovascular or respiratory disease or conditions.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.5 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.6-9 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,10 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.2 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/.
References
- Estimate: Masimo data on file.
- As ranked in U.S. News and World Report. health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; Jan 8;338.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Hospital Stork™ and SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results;risks related to our belief that Masimo's unique technologies, including Stork and SET®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to the assumption that Stork OTC monitoring and alarm features will be available through the Masimo Stork App and Stock OTC bundles will be available online and at retailers this summer; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Matt Whewell
Phone: (720)-838-0691
Email: Matt.whewell@masimo.com

Masimo Founder and CEO Joe Kiani to Speak on AI at Global Patient Safety Summit in Chile
Kiani’s Speech on Wednesday, at the 6th Global Ministerial Summit on Patient Safety, Will Explore How AI-Based Medical Solutions Can Empower Clinicians and Improve Patient Safety
Masimo (NASDAQ: MASI) today announced that its Founder and CEO, Joe Kiani, will be a featured speaker tomorrow, April 17, at the 6th Global Ministerial Summit on Patient Safety. Kiani’s speech, “Empowering Patient Safety Through AI,” will discuss how artificial intelligence-based technologies, by analyzing both historical and immediate data, can help clinicians prevent errors of omission, improving patient safety, patient outcomes, and clinician efficiency.
As Kiani will note, Masimo’s AI-powered medical technologies, including those used as part of the Masimo Hospital Automation™ family of solutions available today, can ease the burden of staffing shortages by improving clinician efficiency. Furthermore, by integrating and analyzing patient data from a variety of sources, in real time, such technology can provide greater situational awareness than alarms alone and help predict increases in patient risk, prompting clinicians to intervene sooner to catch physiological deterioration before a patient’s condition worsens.
“We introduced the Halo engine in 2010,” said Kiani. “Halo is the first predictive algorithm to incorporate learnings from expert clinicians to identify deterioration patterns in multiple physiological parameters simultaneously in real time to help clinicians identify patient safety issues before it’s too late. Without AI, these types of solutions would not be possible. We’re proud to be at the forefront of this very promising avenue of improving patient safety and patient care. AI – when implemented with the ingenuity, integrity, and expertise Masimo is known for – has the predictive power to help clinicians shape robust streams of patient data – both historical and immediate – into meaningful, actionable insights about their patients. Tools such as Halo ION® and the newly introduced Opioid Halo™, soon to be joined by Sepsis Halo, Activity Halo, and more, can help clinicians prioritize patient needs, make more informed decisions and more efficient diagnoses, and streamline workflows in numerous ways. At Masimo, we have always championed patient safety, and we’re particularly excited to collaborate with caregivers around the world to advance patient safety using AI.”
For more information about the Global Ministerial Summit, please visit psschile.minsal.cl. For more information about Masimo and its innovative solutions, please visit www.masimo.com.
Halo, Sepsis Halo, Activity Halo, and AI-based algorithms for Halo ION are not FDA cleared.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.9 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/.
RPVi has not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results;risks related to our belief that Masimo's unique technologies contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks that Masimo may not realize the expected benefits and goals that may be achieved by its collaboration with UCHealth; risks that Masimo and UCHealth fail to collaborate in the areas stated in this press release as planned; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: elamb@masimo.com

Masimo Named One of Fast Company’s Most Innovative Companies in North America
Masimo (NASDAQ: MASI), a global leader in noninvasive monitoring technologies and audio products, is thrilled to announce its recognition as one of Fast Company’s Most Innovative Companies in North America for 2024. This prestigious accolade is especially meaningful given Masimo’s notable breakthroughs and the company’s commitment to improve people’s lives through innovation.
“We are honored to be recognized by Fast Company as one of the top 10 Most Innovative Companies in North America for 2024,” said Joe Kiani, Founder and CEO of Masimo. “This recognizes our ongoing commitment to driving innovation to improve lives. Since our founding 35 years ago, we have made solving what others thought unsolvable our task, and fortunately, thanks to our incredible team, we have solved many ‘unsolvable problems.’ Our solutions have helped improve patient care and reduce the cost of care. We intend to continue to innovate to usher in 22nd century healthcare in this decade.”
Masimo’s inclusion in this respected list highlights its unwavering dedication to innovation and the transformative impact of its technologies in the healthcare sector. Masimo has been recognized as one of the 10 most innovative organizations in the North America sector, a testament to its leadership in advancing patient care through cutting-edge and innovative solutions. In their announcement, Fast Company highlighted several Masimo breakthrough technologies that received FDA clearance in 2023, including Opioid Halo™, an opioid overdose and prevention solution that is the first and only FDA-authorized device to alert you in the event of an opioid overdose; Stork™, a revolutionary baby monitor that provides continuous, accurate monitoring, with alarms, based on Masimo’s foundational pulse oximetry technology; and Masimo W1™, a first-of-its-kind wearable offering accurate, continuous health data and now available both in a consumer sport-oriented version and as a medical watch – the first FDA-cleared watch to provide continuous real-time oxygen saturation and pulse rate for over-the-counter and prescription use.
From its inception, Masimo has been at the forefront of developing innovative monitoring technologies and hospital automation solutions that empower healthcare professionals to make informed decisions and improve patient outcomes. This is why over 200 million people are monitored with Masimo pulse oximeters annually and 9 out of the top 10 hospitals in the United States rely on Masimo SET® pulse oximeters.1-2 Today, Masimo’s portfolio encompasses a wide range of cutting-edge healthcare solutions, including its flagship Signal Extraction Technology® (SET®) pulse oximeter, Bridge™ for opioid withdrawal symptom relief, PerL with Masimo AAT™ for personalized listening, and MightySat® Medical, the first and only FDA-cleared medical fingertip pulse oximeter available over the counter. In addition, Masimo’s most recent consumer products, such as Masimo W1, Stork, and Opioid Halo, provide solutions for continuous and accurate data tracking, so individuals can take better control of their health.
Masimo’s innovative solutions have revolutionized patient monitoring and have been instrumental in enhancing clinical decision-making, reducing medical errors, and optimizing healthcare delivery. By continually pushing the boundaries of innovation, Masimo remains at the forefront of transforming healthcare and improving the lives of patients worldwide.
For more information about Masimo and its innovative solutions, please visit www.masimo.com.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.3 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,4 improve CCHD screening in newborns,5 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.6-9 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,1 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.2 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/. RPVi has not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Estimate: Masimo data on file.
- health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
About Fast Company
Fast Company is the only media brand fully dedicated to the vital intersection of business, innovation, and design, engaging the most influential leaders, companies, and thinkers on the future of business. Headquartered in New York City, Fast Company is published by Mansueto Ventures LLC, along with our sister publication Inc., and can be found online at www.fastcompany.com.
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique technologies, including Masimo SET®, contribute to positive clinical outcomes and patient safety; risks that the researchers’ conclusions and findings may be inaccurate; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Matt Whewell
Phone: (720)-838-0691
Email: Matt.whewell@masimo.com

Masimo Celebrates Multiple Wins at the iF DESIGN AWARDS 2024
Masimo (NASDAQ: MASI) is proud to celebrate multiple wins across various categories at the prestigious iF DESIGN AWARDS 2024, showcasing its commitment to excellence in design and innovation throughout diverse industries.
With an impressive 11,000 submissions from 72 countries, the annual awards are organized by the esteemed iF International Forum Design GmbH in Hanover, Germany, and recognize outstanding achievements in design from around the world.
Under the Masimo umbrella, the following products have been honored with the coveted iF DESIGN AWARD seal:
- Masimo Stork™ – Baby Monitoring System (Baby/Kids category)
- Bowers & Wilkins Px7 S2e – Over-Ear Wireless Noise Canceling Headphones (Audio category)
- Bowers & Wilkins Px8 – Over-Ear Wireless Noise Canceling Headphones (Audio category)
- Bowers & Wilkins for Volvo EM90 – Car Audio System (Automotive category)
- Bowers & Wilkins for Aston Martin DB12 – Car Audio System (Automotive category)
- Bowers & Wilkins for BMW 5 Series – Car Audio System (Automotive category)
These wins underscore Masimo’s commitment to delivering excellence in design and innovation across a wide range of industries, from healthcare to consumer electronics. The recognition from iF DESIGN AWARDS reaffirms Masimo's dedication to pushing the boundaries of design and technology to improve the lives of people around the world.
Joe Kiani, Founder and CEO of Masimo, said, “We are honored to be recognized by iF Design. I’m especially proud that products from both our consumer health and our audio divisions, independently designed and addressing different needs, have been awarded simultaneously – a sign of our pursuit of excellence, innovation, and creativity across the board.”
The winners were selected by a distinguished jury comprising 132 independent experts from various fields, highlighting the global significance of these accolades.
Masimo remains committed to advancing healthcare technologies and enhancing consumer experiences through cutting-edge design and innovation. For more information about Masimo and its award-winning brands, please visit www.masimo.com.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.9 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/.ducts can be found at professional.masimo.com/evidence/featured-studies/feature/.
RPVi has not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results;risks related to our belief that Masimo's unique technologies contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks that Masimo may not realize the expected benefits and goals that may be achieved by its collaboration with UCHealth; risks that Masimo and UCHealth fail to collaborate in the areas stated in this press release as planned; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: elamb@masimo.com

Masimo and UCHealth Announce a Clinical Monitoring Partnership to Improve Care with Telehealth
Masimo (NASDAQ: MASI), a leading global provider of medical technology and automation solutions, and UCHealth, a nationally recognized network of hospitals, clinics, and providers based in Colorado, announced today that they are entering into a strategic collaboration to improve the standard of patient care using the latest in virtual care and telehealth capabilities. With an emphasis on advancing technology-enabled care, including virtual and remote care, the collaboration is designed to leverage the unique capabilities of both organizations and pursue their mutual goals of improving patient outcomes, reducing the cost of care, and transforming models of care delivery across the continuum, both in and outside the hospital.
Joe Kiani, Founder and CEO of Masimo, said, “We are grateful for the opportunity to collaborate with world-class organizations like UCHealth. By combining our technological expertise and their clinical expertise, together we can foster a new generation of healthcare solutions that improve the lives of patients and clinicians alike – ultimately advancing the forefront of care.”
“UCHealth is committed to driving medical advances through clinical innovation, pioneering research and world-class education. Our commitment aligns with Masimo’s vision for achieving tomorrow’s outcomes and helping institutions like ours improve the ways we monitor physiological status across more care areas than ever before, and extend high-quality care beyond the hospital and into the home by harnessing the power of data science and sensors,” said Dr. Richard Zane, Chief Innovation Officer at UCHealth and Chair of Emergency Medicine at the University of Colorado School of Medicine. “We are particularly excited by Masimo’s innovations in telehealth, virtual and remote care, and how they can help us support improved outcomes for our patients and for people everywhere.”
Opportunities for collaboration between Masimo and UCHealth may include:
- Offering innovative virtual care and remote monitoring solutions, through the optimization of enterprise patient monitoring technologies, patient flow management, and advanced analytics.
- Augmenting UCHealth’s expertise in evidence-based clinical workflows with Masimo virtual care solutions.
- Collaborating on innovative direct-to-consumer (D2C) and provider-to-provider (P2P) services, to introduce virtual clinical services focusing on episodic and chronic care management.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.9 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/.
RPVi has not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
About UCHealth
UCHealth is an innovative, nonprofit health system that delivers the highest quality medical care with an excellent patient experience. UCHealth includes 33,000 employees, 14 acute-care hospitals and hundreds of physicians across Colorado, southern Wyoming and western Nebraska. With University of Colorado Hospital on the CU Anschutz Medical Campus as its academic anchor and the only adult academic medical center in the region, UCHealth is dedicated to providing unmatched patient care in the Rocky Mountain West. Offering more than 200 clinic locations, UCHealth provides extensive community benefits and pushes the boundaries of medicine through advanced treatments and clinical trials, improving health through innovation. UCHealth’s CARE Innovation Center collaborates with leading industry and start-up partners to fundamentally transform the way health care is delivered.
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results;risks related to our belief that Masimo's unique technologies contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks that Masimo may not realize the expected benefits and goals that may be achieved by its collaboration with UCHealth; risks that Masimo and UCHealth fail to collaborate in the areas stated in this press release as planned; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: elamb@masimo.com Media Contact
UCHealth
Kelli Christensen
Phone: (720) 848-5809
Email: Kelli.Christensen@uchealth.org

Masimo and UCHealth Announce a Clinical Monitoring Partnership to Improve Care with Telehealth
Masimo (NASDAQ: MASI), a leading global provider of medical technology and automation solutions, and UCHealth, a nationally recognized network of hospitals, clinics, and providers based in Colorado, announced today that they are entering into a strategic collaboration to improve the standard of patient care using the latest in virtual care and telehealth capabilities. With an emphasis on advancing technology-enabled care, including virtual and remote care, the collaboration is designed to leverage the unique capabilities of both organizations and pursue their mutual goals of improving patient outcomes, reducing the cost of care, and transforming models of care delivery across the continuum, both in and outside the hospital.
Joe Kiani, Founder and CEO of Masimo, said, “We are grateful for the opportunity to collaborate with world-class organizations like UCHealth. By combining our technological expertise and their clinical expertise, together we can foster a new generation of healthcare solutions that improve the lives of patients and clinicians alike – ultimately advancing the forefront of care.”
“UCHealth is committed to driving medical advances through clinical innovation, pioneering research and world-class education. Our commitment aligns with Masimo’s vision for achieving tomorrow’s outcomes and helping institutions like ours improve the ways we monitor physiological status across more care areas than ever before, and extend high-quality care beyond the hospital and into the home by harnessing the power of data science and sensors,” said Dr. Richard Zane, Chief Innovation Officer at UCHealth and Chair of Emergency Medicine at the University of Colorado School of Medicine. “We are particularly excited by Masimo’s innovations in telehealth, virtual and remote care, and how they can help us support improved outcomes for our patients and for people everywhere.”
Opportunities for collaboration between Masimo and UCHealth may include:
- Offering innovative virtual care and remote monitoring solutions, through the optimization of enterprise patient monitoring technologies, patient flow management, and advanced analytics.
- Augmenting UCHealth’s expertise in evidence-based clinical workflows with Masimo virtual care solutions.
- Collaborating on innovative direct-to-consumer (D2C) and provider-to-provider (P2P) services, to introduce virtual clinical services focusing on episodic and chronic care management.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.9 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/.
RPVi has not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
About UCHealth
UCHealth is an innovative, nonprofit health system that delivers the highest quality medical care with an excellent patient experience. UCHealth includes 33,000 employees, 14 acute-care hospitals and hundreds of physicians across Colorado, southern Wyoming and western Nebraska. With University of Colorado Hospital on the CU Anschutz Medical Campus as its academic anchor and the only adult academic medical center in the region, UCHealth is dedicated to providing unmatched patient care in the Rocky Mountain West. Offering more than 200 clinic locations, UCHealth provides extensive community benefits and pushes the boundaries of medicine through advanced treatments and clinical trials, improving health through innovation. UCHealth’s CARE Innovation Center collaborates with leading industry and start-up partners to fundamentally transform the way health care is delivered.
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results;risks related to our belief that Masimo's unique technologies contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks that Masimo may not realize the expected benefits and goals that may be achieved by its collaboration with UCHealth; risks that Masimo and UCHealth fail to collaborate in the areas stated in this press release as planned; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: elamb@masimo.com Media Contact
UCHealth
Kelli Christensen
Phone: (720) 848-5809
Email: Kelli.Christensen@uchealth.org

Masimo Announces the First FDA-cleared “Over-the-Counter” Fingertip Pulse Oximeter
Masimo MightySat® Medical Provides Clinically Proven Masimo SET® Pulse Oximetry to Consumers for Medical Use Without a Prescription
Masimo (NASDAQ: MASI) announced today FDA clearance of MightySat® Medical, making it the first and only FDA-cleared medical fingertip pulse oximeter available Over-The-Counter (OTC) direct to consumers without a prescription. All other pulse oximeters available at drug stores and online and sold direct to consumers are not FDA cleared and should not be relied upon for medical use; many of them have been reported to give erroneous measurements.1 This clearance brings consumers a pulse oximeter medical device powered by Masimo SET® pulse oximetry—the same technology relied on by hospitals and clinics around the world to monitor more than 200 million patients every year2 and shown to have no clinically significant difference in accuracy or bias between light- and dark-skinned individuals.3 Starting today, the MightySat Medical Pulse Oximeter can be purchased at masimo.com, and it will soon be available for purchase at retail and drug stores across the country.
Joe Kiani, Founder and CEO of Masimo, said, “Until now, consumers and even healthcare providers had no way of knowing what pulse oximeter they could trust to use at home. On the internet and even in drug stores, they are inundated with a myriad of products that are unreliable, with misleading advertisements about their abilities to provide accurate measurements of oxygen saturation and pulse rate. This clearance of MightySat Medical for consumers eliminates the confusion, placing an FDA-cleared, accurate, reliable, and revolutionary SET® pulse oximeter, with technology that hospitals have been using for more than 25 years, directly into their hands. Healthcare providers can also now be confident when referring their patients to get MightySat Medical knowing that it has actually been cleared by the FDA as an OTC medical pulse oximeter.”
In 2021, the FDA issued a safety communication clarifying that it does not review the accuracy of “health and wellness” pulse oximeters and highlighting the common limitations of both health and wellness and some prescription pulse oximeters—such as difficulty measuring accurately during patient motion, on patients with poor circulation (low perfusion), and on darker skin pigmentation.4 The overwhelming majority of consumers say that device accuracy—measurements they can trust—is their top priority when choosing a pulse oximeter. MightySat Medical provides a direct-to-consumer option for a medical device with hospital-grade pulse oximetry technology, Masimo SET®, whose performance has been reviewed and cleared by the FDA for medical use. MightySat Medical provides accurate and reliable oxygen saturation (SpO2) and pulse rate (PR) measurements on patients of all skin colors and patients with low perfusion.
Consumers with diagnosed breathing problems or lung diseases such as asthma, chronic obstructive pulmonary disease (COPD), lung cancer, flu, pneumonia, or COVID-19 depend on accurate assessment of their arterial blood oxygen saturation that they can confidently share with their healthcare providers. Prior to the FDA clearance of MightySat Medical, consumers were only able to buy unregulated health and wellness devices without a prescription. Now, MightySat Medical with Masimo SET® provides an FDA-cleared medical device with validated accuracy without the need for a prescription. SET®, available in numerous hospital devices, is the primary pulse oximetry technology at 9 of the top 10 U.S. hospitals listed in the U.S. News and World Report Honor Roll.5 In addition, over 100 independent and objective studies have shown that SET® outperforms other pulse oximetry technologies.6
Peter Pronovost, MD, PhD, Chief Quality and Clinical Transformation Officer, University Hospitals, Cleveland, commented, “More and more care is moving to the patient’s home. Patients now have the ability to monitor their oxygen levels and pulse rate, which can help them know when they need to seek care. Yet to be useful, their pulse oximetry data must be accurate and the accuracy of these devices varies widely. Doctors and nurses rely on Masimo technology in the hospital.”
Masimo MightySat Medical is intended for the spot-checking of functional oxygen saturation of arterial hemoglobin (SpO2) and pulse rate (PR).
MightySat Medical is indicated for use with individuals 18 years and older who are well or poorly perfused under no motion conditions.
MightySat Medical is not intended for the diagnosis or screening of lung disease. Treatment decisions using the device should only be made under the advice of a healthcare provider.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.6 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,7 improve CCHD screening in newborns,8 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.9-12 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,2 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.5 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/.RPVi has not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Lipnick M, Feiner J, Au P, Bernstein M, Bickler P. The Accuracy of 6 Inexpensive Pulse Oximeters Not Cleared by the Food and Drug Administration: The Possible Global Public Health Implications. Anesth Anal. Aug 2016. 123(2)338-345. DOI: 10.1213/ANE.0000000000001300.
- Estimate: Masimo data on file.
- Barker SJ, Wilson WC. Racial effects on Masimo pulse oximetry: a laboratory study. J Clin Monit Comput. 2023 Apr;37(2):567-574. DOI: 10.1007/s10877-022-00927-w.
- www.fda.gov/medical-devices/safety-communications/pulse-oximeter-accuracy-and-limitations-fda-safety-communication
- health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness ofMasimo MightySat® and SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results;risks related to our belief that Masimo's unique technologies, including includingMasimo MightySat and SET®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: elamb@masimo.com

New Peer-Reviewed Study Finds That Masimo SET® Pulse Oximetry Measures Accurately on Both Black and White People Even During Low Perfusion
Masimo (NASDAQ: MASI), today announced the findings of a retrospective, peer-reviewed study published in the Journal of Clinical Monitoring and Computing in which Dr. Vikrant Sharma, along with Dr. Steven J. Barker, Dr. William C. Wilson, and colleagues at Masimo performed a focused analysis of previously published data to evaluate the impact of low perfusion on the performance of Masimo SET® pulse oximetry across a variety of skin pigmentations.1 The analysis demonstrated that Masimo RD SET® sensors accurately measured oxygen saturation (SpO2) for both Black and White subjects when perfusion index (Pi) was normal and when Pi was low – adding to the body of evidence that Masimo SET® pulse oximetry delivers accurate values across the skin tone range, with no clinically significant difference in accuracy or bias, even in challenging conditions.
In a study published in 2023, Drs. Barker and Wilson analyzed Masimo laboratory data obtained from self-identified Black and White volunteer subjects to evaluate differences in Masimo pulse oximetry accuracy and bias on the basis of skin tone. To do so, they reviewed more than 7,000 paired data samples (collected between 2015 and 2021) from 75 subjects (39 Black and 36 White) and found no clinically significant difference in accuracy or bias.2
For this newly published study, noting that low peripheral perfusion is a “recognized confounder of conventional pulse oximetry” and in light of concerns that low perfusion combined with dark skin pigmentation might decrease pulse oximetry’s accuracy, the investigators sought to determine whether accuracy on Black or White subjects was impacted by a subject’s perfusion index (Pi) with Masimo SET® pulse oximetry. To that end, they abstracted Pi values from their dataset, and divided them into “low perfusion” (Pi ≤ 1) and “normal perfusion” (Pi > 1) groups. They then performed statistical analyses to determine bias (the mean difference between SpO2 and arterial oxygen saturation [SaO2]), precision (the standard deviation of the difference), and accuracy (root-mean-square error, or ARMS*).
The researchers found that in the normal perfusion group, comparing SpO2 to SaO2 values, there was overall bias and precision of +0.18% ± 1.34%, with accuracy of 1.37% ARMS. For the subset of Black subjects, there was bias and precision of -0.26% ± 1.37%, and for White subjects, -0.12% ± 1.31%. In the low perfusion group, there was overall bias and precision of 0.48% ± 1.59%, with accuracy of 1.64% ARMS. For Black subjects, bias and precision were 0.19% ± 1.53%, and for White subjects, 0.91% ± 1.57%.
Based on their analysis, the authors concluded, “Masimo SET® pulse oximeters with RD SET® sensors are accurate for individuals of both Black and White races when Pi is normal, as well as during conditions when Pi is low. The ARMS for all conditions studied is well within FDA standards. This study was conducted in healthy volunteers during well-controlled laboratory desaturations, and results could vary under certain challenging clinical conditions.”
However, the authors noted that controlling conditions in the laboratory setting helps “minimize confounders that are present in clinical scenarios, allowing for greater focus on the topics of skin tone and Pi. Indeed, abnormal hemoglobin species (e.g., carboxyhemoglobin and methemoglobin) [known clinical SpO2 confounders] were measured and reported in the earlier paper by Barker and Wilson, and the values were similar (statistically the same) between Black and White groups.2 Also, one can only ethically conduct desaturation studies using healthy volunteer subjects in a safe setting.”
*ARMS accuracy is a statistical calculation of the difference between device measurements and reference measurements. Approximately two-thirds of the device measurements fell within +/- ARMS of the reference measurements in a controlled study.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.3 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,4 improve CCHD screening in newborns,5 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.6-9 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,10 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.11 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/. RPVi has not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Sharma V, Barker S, Sorci R, Park L, Wilson W. Racial effects on Masimo pulse oximetry: impact of low perfusion index. J Clin Monit Comput. 19 Jan 2024. DOI: 10.1007/s10877-023-01113-2.
- Barker SJ, Wilson WC. Racial effects on Masimo pulse oximetry: a laboratory study. J Clin Monit Comput. 2023 Apr;37(2):567-574. DOI: 10.1007/s10877-022-00927-w.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique technologies, including Masimo SET®, contribute to positive clinical outcomes and patient safety; risks that the researchers’ conclusions and findings may be inaccurate; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: elamb@masimo.com

Masimo Announces Reinstatement of Import Ban on Infringing Apple Watches
Masimo (NASDAQ: MASI), a global leader in innovative noninvasive monitoring technologies, welcomes the Federal Circuit’s ruling to lift the temporary stay on the import ban of certain Apple Watch models. This decision reinstates the U.S. International Trade Commission’s import ban and cease and desist order on Apple watches that were found to infringe Masimo’s patented pulse oximetry technology.
“The Federal Circuit's decision to lift the temporary stay is a victory for the integrity of the American patent system and the safety of people relying on pulse oximetry,” said Joe Kiani, Founder and CEO of Masimo. “It affirms that even the largest and most powerful companies must respect the intellectual rights of American inventors and must deal with the consequences when they are caught infringing others’ patents.”
Masimo has previously made available a study showing that Apple Watch’s pulse oximetry missed over 90% of potentially life-threatening events. The Apple Watch pulse oximeter was not cleared by the United Stated Food and Drug Administration for medical use. On the other hand, the Masimo W1® health watch was recently cleared by the FDA for its indicated medical uses, including continuous pulse oximetry. Masimo’s pulse oximetry technology is used on over 200 million patients in hospitals a year1 and has been proven to help save babies’ eyesight in the neonatal ICU,2 detect critical congenital heart defects in newborns,3 and save the lives of patients in post-surgical wards who are taking opioids.4-7
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.8 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.9 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97® and the Masimo W1® medical watch. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and Masimo W1 Sport. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature.RPVi has not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Estimate: Masimo data on file.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements - Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness ofMasimo W1®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results;risks related to our belief that Masimo's unique technologies, including includingMasimo W1, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Masimo
Evan Lamb
Phone: (949) 396-3376
Email: elamb@masimo.com