Home / Bridge
Bridge
Bridge™
Bridge™
Drug-free Opioid Withdrawal Device
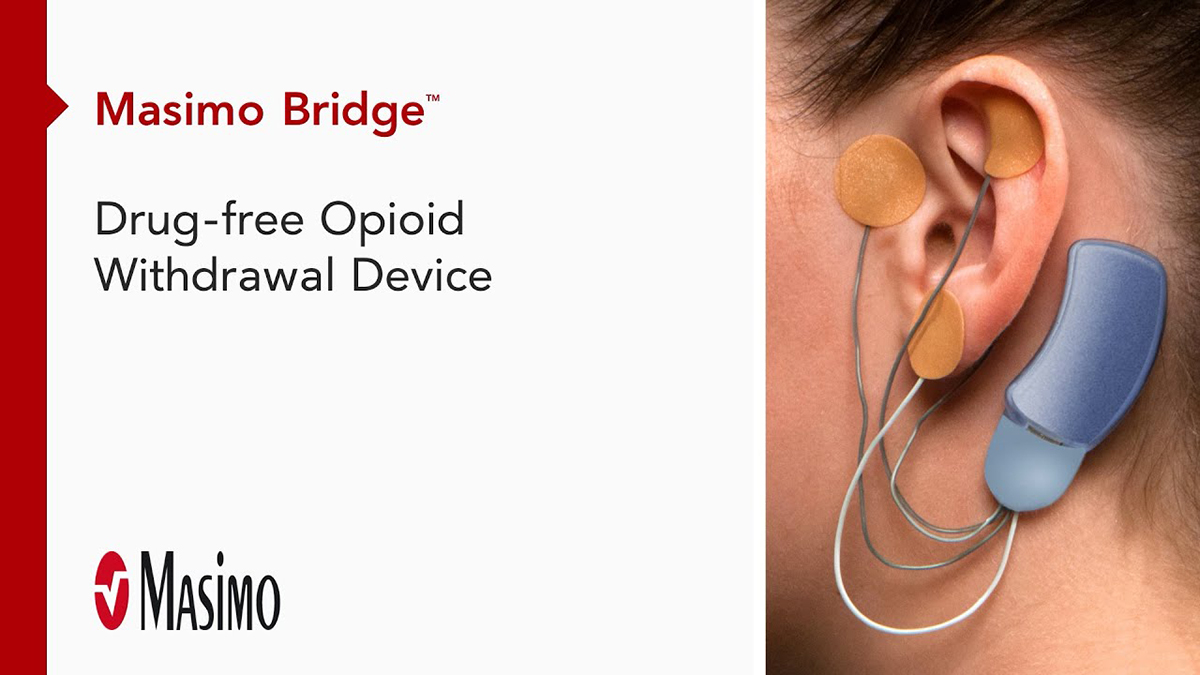
Bridge™
Drug-Free Opioid Withdrawal Device
The first FDA-cleared medical device for the reduction of opioid withdrawal symptoms,* Bridge is designed to give your patients the hope they need to continually progress through treatment—without the fear of withdrawal.
Proven to Provide Relief
Proven to Provide Relief
FAST
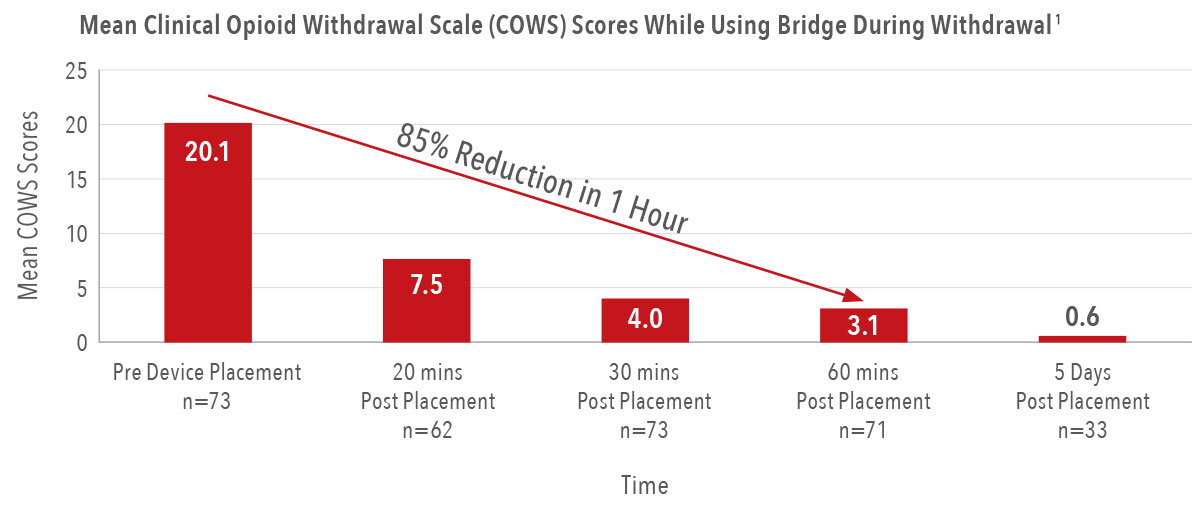
85%
reduction in the severity of a patient’s symptoms in the first hour of placement, with the first signs of relief in as few as 20 minutes1
EFFECTIVE
88%
of patients successfully transitioned into a medication-assisted treatment (MAT) program while using Bridge1
97%
reduction in reported withdrawal symptom severity by the fifth day of wear1
SAFE
0
reported side effects with minimal risks during device placement2
How Does Bridge Work?
How Does Bridge Work?
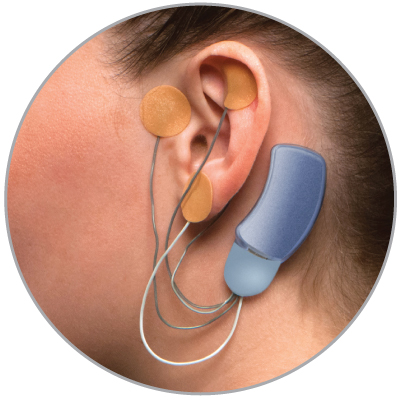
Bridge is a small electrical nerve stimulator device that contains a battery-powered chip and wires that are applied around a patient's ear in a short, non-surgical, in-office procedure, providing five days of continuous relief.†
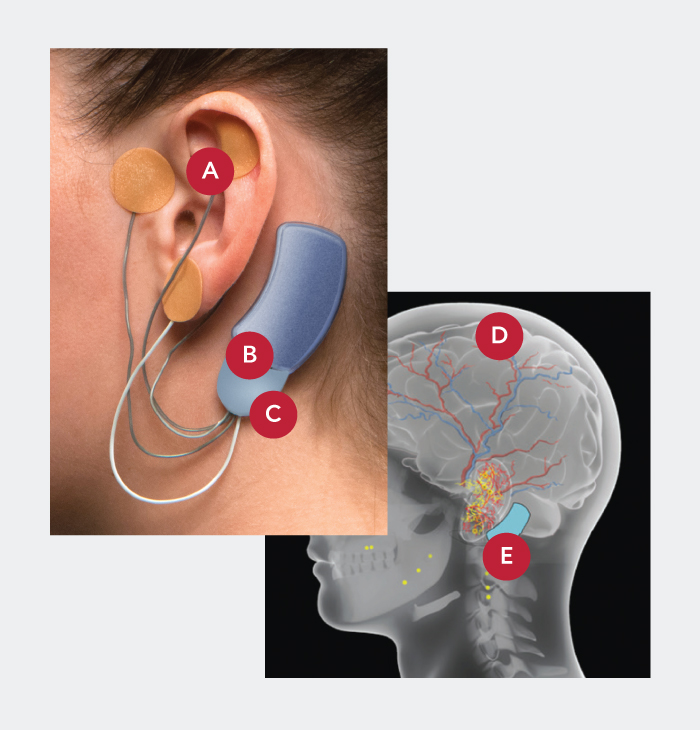
- Applied by a qualified healthcare professional in a short, non-surgical, in-office procedure
- Fits comfortably behind the ear
- Provides five days of continuous relief
- Stimulated nerves transmit these impulses to the brain, reducing withdrawal symptoms
- Sends gentle electrical impulses through wires to nerves around the ear
Hear What Others Are Saying About Bridge
Hear What Others Are Saying About Bridge

“Bridge helped us reduce Opioid Detox AMA rates by 50%.”
Bridge helped us reduce Opioid Detox AMA rates by 50%. Opioid users who are chemically dependent desire a powerful, timely solution, and Bridge delivers just that. We were astounded by how well the device worked and now use it to reduce recidivism and reduce abandonment of treatment.
Jeffrey A. Thomas, MHS, LPC, CAADC / Chief Executive Officer, White Deer Run
“It was like all the anxiety just left his body.”
That was the first time I saw Bridge get put on a patient. This guy was a nervous wreck and within seconds of our medical assistant putting it on him he fell asleep sitting up in the chair. I got scared and woke him up but he said it was an amazing experience. It was like all the anxiety just left his body. Immediately. This guy has been using EVERY HOUR. I kid you not. I've never heard of someone using that much and that frequently. He was supposed to get it put on then go to detox but he didn't end up going to detox today. He called his mom to come get him. He went home and ate spaghetti with his mom and got along with her for the first time in years. He says he will go to detox tomorrow but maybe he will end up doing this outpatient? That was crazy. I had to let you know.
Lauren R., Program Director / MATClinics
“I think we all felt like we were watching a miracle.”
The story from this week is incredible. The individual was sitting on a chair in the shower, and she collapsed in the shower off of the chair and a medical response was called. She appeared to be having a seizure however her body was really just locked up. She was in so much pain her toes were literally curling up and was defecating on herself. She was gray in the face and was in really bad shape. Our deputies immediately took her to medical where they placed a Bridge device on her and literally within 20 minutes she was up and moving, with color back in her face. She had a little queasiness still but that is literally it. I think we all felt like we were watching a miracle. Thank you so much for introducing us to the Bridge.
Bailey H., Inmate Rehabilitations Programs Manager / Chesterfield County Jail
Who Can Bridge Help?
Who Can Bridge Help?

Bridge is designed to help patients‡ with opioid withdrawal symptoms. Opioid withdrawal symptoms have been found to occur while:
- Initiating medication-assisted treatment (MAT)
- Transitioning from opioid agonist to antagonist treatment
- Tapering off of MAT following prolonged therapy
- Tapering off opioid therapy
- Tapering off opioid pain treatment, when dependence is present
Try Bridge Today
Try Bridge Today
See how Bridge can help patients progress to the next phase of recovery.
Contact us today for an in-office demonstration.
References:
* Bridge is FDA cleared for the treatment of opioid withdrawal symptoms only. It is not intended to be used to directly treat opioid use disorders.
† Bridge requires a prescription and is offered to qualified healthcare professionals with training. Bridge includes a kit containing everything required to apply the device.
‡ Contraindications: Use of cardiac pacemakers, Hemophilia, Psoriasis vulgaris
1Miranda A, Taca A. Neuromodulation with percutaneous electrical nerve field stimulation is associated with reduction in signs and symptoms of opioid withdrawal: a multisite, retrospective assessment [published correction appears in Am J Drug Alcohol Abuse. 2018;44(4):498]. Am J Drug Alcohol Abuse. 2018;44(1):56‐63. doi:10.1080/00952990.2017.1295459
2Roberts, Arthur et al. “Minimal adverse effects profile following implantation of periauricular percutaneous electrical nerve field stimulators: a retrospective cohort study.” Medical devices (Auckland, N.Z.) vol. 9 389-393. 3 Nov. 2016, doi:10.2147/MDER.S107426Roberts, Arthur et al. “Minimal adverse effects profile following implantation of periauricular percutaneous electrical nerve field stimulators: a retrospective cohort study.” Medical devices (Auckland, N.Z.) vol. 9 389-393. 3 Nov. 2016, doi:10.2147/MDER.S107426.
RESOURCES
Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. See instructions for use for full prescribing information, including indications, contraindications, warnings, and precautions.
PLCO-007207/PLM-12507F-0324