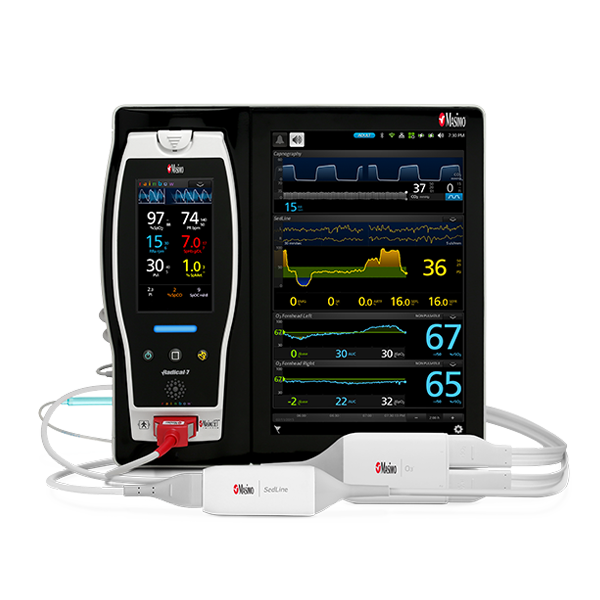
Newborn Care
Advanced Technology For When Every Second Counts
Home / Newborn Care
Newborn Care
Timely, reliable monitoring is vital when trying to accurately assess a newborn’s oxygenation status at birth and when screening for congenital heart defects. Committed to improving care for this fragile patient population, Masimo has designed sensors and monitoring solutions specifically for newborns that provide clinicians with pertinent physiological data quickly and efficiently – without sacrificing accuracy.
Masimo SET® Measure-through Motion and Low Perfusion™ Pulse Oximetry
Masimo SET® Measure-through Motion and Low Perfusion™ Pulse Oximetry
Masimo’s breakthrough Signal Extraction Technology® (SET®) overcomes the limitations of conventional pulse oximetry with the ability to measure through motion and low perfusion. Two separate studies found that Masimo SET® pulse oximeters detected approximately 10 times more true events than other “Next Generation” pulse oximeters studied.1,2
Performance During Motion and Low Perfusion3
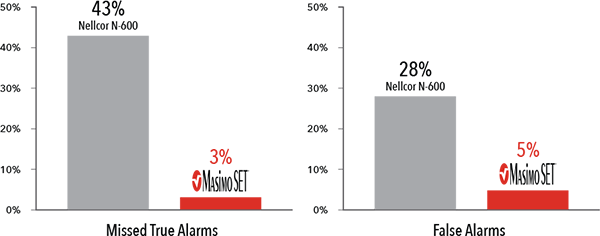
Masimo SET® had 3% missed true alarms and 5% false alarms versus 43% and 28%, respectively, using competitor technology.3
Results shown are calculated by combining sensitivity and specificity outcomes of machine-generated and volunteer-generated motion.
In another study comparing the ability of three pulse oximetry technologies to detect hypoxic events, Masimo SET® pulse oximetry demonstrated the highest sensitivity and specificity during induced conditions of motion and low perfusion.3
Solutions for Newborn Resuscitation
Solutions for Newborn Resuscitation

Newborn Neonatal SpO2 sensors automatically configure Masimo SET® pulse oximeters for the fastest response time and maximum sensitivity settings, allowing clinicians to focus on patient care during newborn resuscitation.
![]() Velaid SofTouch™ design allows for quick application and repositioning on newborn skin.
Velaid SofTouch™ design allows for quick application and repositioning on newborn skin.
Improving CCHD Screening with Masimo SET®
Improving CCHD Screening with Masimo SET®
Masimo SET® was the first pulse oximetry technology to receive FDA 510(k) clearance in the labeling to screen newborn patients for critical congenital heart disease (CCHD).5
To date, there have been five published CCHD screening studies, representing a total of 241,239 infants, that used exclusively Masimo SET® pulse oximeters and sensors6-10, two of which (59,876 subjects) were the basis for the CCHD workgroup recommendation for CCHD screening protocols.8,9,11 These studies, all using Masimo SET®, concluded that pulse oximetry, in conjunction with clinical assessment, improved screening sensitivity compared to routine physical exam alone.6-10
Masimo SET® Sensors for CCHD Screening
Masimo SET® Sensors for CCHD Screening
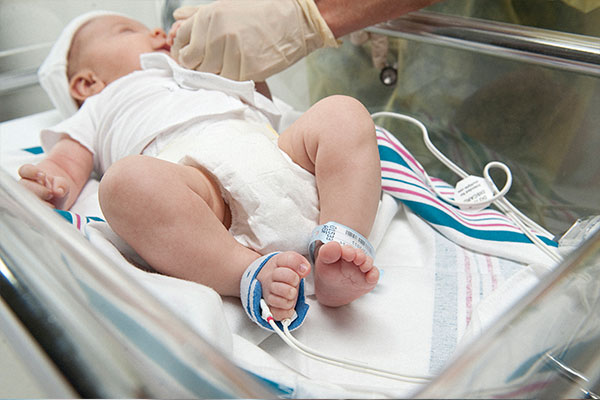
Multisite Y-I reusable sensors with soft foam wraps – which have been used in multiple prominent CCHD screening studies6-10 - enable quick, comfortable application and removal, without disturbing the patient.

RD SET™ Neo single-patient-use, wrap-around style sensors feature a flat, lightweight cable that lies comfortably on the patient’s hand or foot.
Measurements
Measurements
Newborn Care Solutions
References:
- 1.
Hay WW. J of Perinatol, 2002;22:360-36.
- 2.
Barker SJ. Anesth Analg. 2002;95(4):967-72.
- 3.
Shah N. et al. J Clin Anesth. 2012 Aug;24(5):385-91.
- 4.
American Academy of Pediatrics (AAP). (2016). Textbook of Neonatal Resuscitation, 7th Ed.
- 5.
Masimo 510(k) Summary - K120657
- 6.
de-Wahl Granelli A et al. Acta Paediatr. 2007 Oct;96(10):1455-9.
- 7.
Meberg A et al. Pediatr. 2008 Jun;152(6):761-5.
- 8.
de-Wahl Granelli A et al. BMJ. 2009 Jan 8;338:a3037.
- 9.
Ewer AK et al. Lancet. 2011 Aug 27;378(9793):785-94.
- 10.
Zhao QM et al. Lancet. 2014 Aug 30;384(9945):747-54.
- 11.
Kemper AR et al. Pediatrics. 2011 Nov;128(5):e1259-67.
RESOURCES
Rad-97 NIBP is not licensed for sale in Canada.
Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. See instructions for use for full prescribing information, including indications, contraindications, warnings, and precautions.
PLCO-001785/PLM-11094B-0318