Home / Newborn Care / CCHD Screening
CCHD Screening
Critical Congenital Heart Disease
Critical Congenital Heart Disease
Congenital heart defects (CHDs) are the most common birth-related malformation.1 Critical CHDs (CCHDs) are life threatening and account for nearly 25% of all CHDs.2 Babies sent home with an undetected heart defect are at risk of serious complications within the first few days or weeks of life, requiring emergency care,3 and even at risk of dying.
Improved Critical Congenital Heart Disease Screening Sensitivity vs. Clinical Assessment Alone5
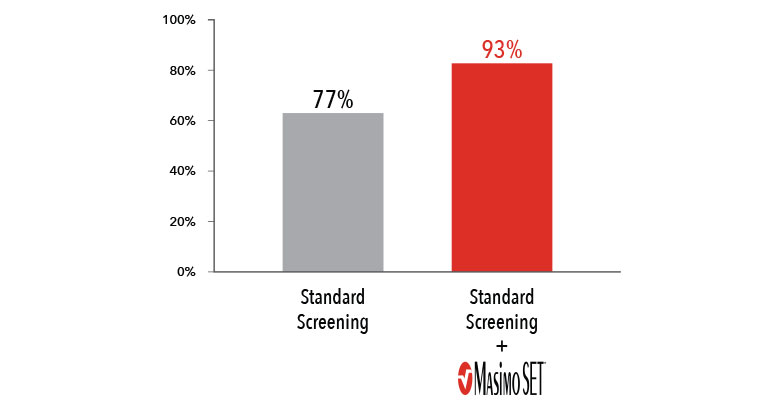
When combined with clinical assessment, Masimo SET® improved CCHD screening sensitivity to 93%5
To date, there have been five published CCHD screening studies that exclusively used Masimo SET® pulse oximeters and sensors4,5,13-15, two of which (59,876 subjects) were the basis for the CCHD workgroup recommendation for CCHD screening protocols.4,7,15 These five studies represent a total of 241,239 infants and include the largest CCHD screening study to date, with over 122,738 subjects.5
These studies, all using Masimo SET®, concluded that pulse oximetry, in conjunction with clinical assessment, improved screening sensitivity compared to routine physical exam alone.4,5,13-15
In addition, Masimo SET® measures perfusion index (Pi). Incorporating perfusion index into screening has been shown to increase sensitivity to the detection of CCHD in infants with pathologically low perfusion.4 In a study of 10,009 infants, when Pi was added to CCHD screening, screening revealed abnormalities in 100% of all newborns who had left heart obstructive disease (LHOD).13
CCHD Screening with Pulse Oximetry
CCHD Screening with Pulse Oximetry
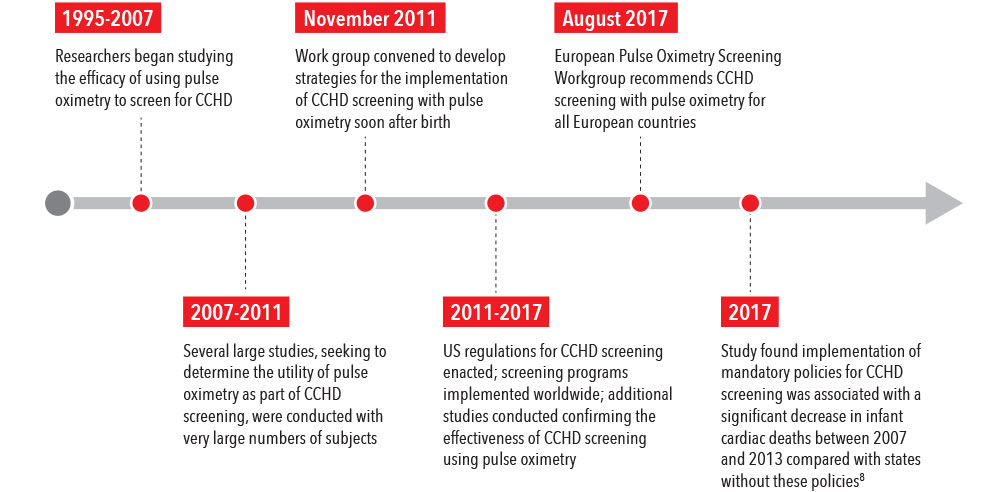
Traditionally, following birth, newborns were observed for evidence of CHDs by physical assessment and monitoring for common symptoms.1 Today, studies show that these methods alone can be unreliable and may fail to detect up to 36% of infants with CCHD before discharge.4,5 Adding screening with pulse oximetry can help diagnose CCHD before an infant becomes symptomatic.6
In 2011, following numerous studies observing the utility of pulse oximetry in screening for CCHD, a work group was convened to develop strategies for the implementation of safe, effective, and efficient CCHD screening with pulse oximetry.7 The work group found sufficient evidence to recommend the use of pulse oximetry to screen for CCHD, and further recommended that screening be performed with motion-tolerant pulse oximeters that report functional oxygen saturation (SpO2) and have been validated in low-perfusion conditions.7
In a recent study, researchers found a 33% decrease in infant cardiac deaths after statewide implementation of mandatory policies for CCHD screening, compared with prior periods and states without policies, as well as a 21% decrease in early infant deaths from other/unspecified cardiac causes.8
Learn More About CCHD Screening with Masimo SET®
Learn More About CCHD Screening with Masimo SET®
References:
- 1.
Ewer AK, et al. NIHR Health Technology Assessment Programme: Executive Summaries. 2012.
- 2.
- 3.
- 4.
de-Wahl Granelli A et al. BMJ. 2009;Jan 8;338.
- 5.
Zhao et al. Lancet. 2014 Aug 30;384(9945):747-54.
- 6.
www.thelancet.com/child-adolescent. Published online August 30, 2017 http://dx.doi.org/10.1016/S2352-4642(17)30066-4
- 7.
Kemper AR et al. Pediatrics. 2011 Nov;128(5):e1259-67.
- 8.
Rahi Abouk et al. JAMA. 2017;318(21):2111-2118. doi:10.1001/jama.2017.17627.
- 9.
Masimo 510(k) Summary - K120657
- 10.
Hay WW. J of Perinatol. 2002;22:360-36.
- 11.
Barker SJ. Anesth Analg. 2002;95(4):967-72.
- 12.
Shah N. et al. J Clin Anesth. 2012 Aug;24(5):385-91.
- 13.
de-Wahl Granelli A et al. Acta Paediatr. 2007 Oct;96(10):1455-9.
- 14.
Meberg A et al. Pediatr. 2008 Jun;152(6):761-5.
- 15.
Ewer AK et al. Lancet. 2011 Aug 27;378(9793):785-94.
RESOURCES
Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. See instructions for use for full prescribing information, including indications, contraindications, warnings, and precautions.
PLCO-005532/PLM-11167C-0122