News & Media-2011
Home / News & Media / 2011
2011
NEWS & MEDIA:

Happy Holidays from Masimo!
Irvine, California — December 21, 2011 — As we honor this very special time of the year and bid farewell to 2011 as a year of great challenge and growth in healthcare, it is with gratitude and a heart-felt desire to give something back to a few of the organizations that share our vision for better care and a better world, in your name. Simply respond with an email to charity@masimo.com specifying your selected charity choice from the list below and we will donate $10 in your name:
 |
We are grateful for the opportunity to affect positive changes that have transformed healthcare and we thank each of you for your support in helping us to make life better for the clinicians and patients we diligently serve.
Cheers to a new year full of great possibilities!
Masimo Mission Statement
Improving patient outcomes and reducing cost of care by taking noninvasive monitoring to new sites and applications.®
Masimo Guiding Principles
- Remain faithful to your promises and responsibilities.
- Thrive on fascination and accomplishment and not on greed and power.
- Make each day as fun as possible.
- Strive to make each year better than the year before, both personally and for the Team.
- Do what is best for patient care.
NOTE: Only e-mails sent to charity@masimo.com from official Livewire members will be processed. Please also include any comments or suggestions you might have that will help us to better fulfill our mission and adhere to our guiding principles.


Masimo to Present at 30th Annual J.P. Morgan Healthcare Conference
IRVINE, Calif., December 20, 2011 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the 30th Annual J.P. Morgan Healthcare Conference at the Westin St. Francis Hotel in San Francisco on Tuesday, January 10, 2012, at 3 p.m. Pacific Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOCT™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


New Clinical Study Presented at the NYPGA Meeting Shows Respiration Rate Monitoring— Considered the Fourth Primary Vital Sign—Has Been Revolutionized With Masimo rainbow Acoustic Monitoring Technology
Irvine, California – December 13, 2011 – Masimo (NASDAQ: MASI) announced today that a new clinical study evaluating Masimo Acoustic Respiration Rate (RRa™)was presented at the New York State Society of Anesthesiologists (NYSSA) Annual Post Graduate Assembly (PGA) Meeting in New York City.
The new study, presented at the 2011 NYPGA, highlights the positive clinical outcomes and patient safety impact of Masimo's unique Acoustic Respiration Rate (RRa™) measurement. Researchers Michael Ramsay, M.D., Elaine Lagow, R.N., and Mohammed Usman, Ph.D., at Baylor University Medical Center in Dallas, Texas, evaluated the accuracy and sensitivity of Masimo RRa and capnography and found that Masimo RRa had higher sensitivity for detecting respiratory pause events compared to capnography. The study, conducted in 34 post-surgical patients in the post-anesthesia care unit (PACU) each monitored for an average of 109 minutes, concluded that RRa provided "acceptable respiration rate accuracy" when compared to capnography, but only RRa had the additional advantage of providing "superior sensitivity for detecting respiratory-pause events" defined as no inspiration or expiration activity for > 30 seconds.1
Performance Characteristics of Masimo RRa and Capnography
(Compared to Reference Method) 1
| Method | Bias (bpm) | Standard deviation (bpm) | Sensitivity = TP/(TP+FN) | Specificity = TN/(TN+FP) |
|---|---|---|---|---|
|
Capnometry |
-0.6 |
2.6 |
62% |
98% |
|
RRa |
-0.1 |
2.4 |
81% |
99% |
Respiration rate is defined as the frequency of breathing expressed as the number of breaths per minute and is considered the fourth primary vital sign for assessing the physiological status of hospitalized patients; however, current methods for respiration rate monitoring are limited by reliability or patient tolerance. In contrast, Masimo rainbow Acoustic Monitoring technology is a breakthrough in respiration rate monitoring that is completely noninvasive, reliable, and virtually unnoticeable to the patient—enabling clinicians to continuously measure patient breathing acoustically to facilitate earlier detection of respiratory compromise and patient distress.
Featuring an innovative adhesive sensor with an integrated acoustic transducer that is applied to the patient's neck to detect upper airway acoustic vibrations on the surface of the skin during the respiratory cycle, Masimo rainbow Acoustic Monitoring uses patented acoustic signal processing that leverages Masimo's revolutionary Signal Extraction Technology (Masimo SET) to separate and process the respiratory signal and display continuous RRa measurements on the Radical-7 Pulse CO-Oximeter. RRa is part of Masimo rainbow®SET, a noninvasive patient monitoring platform enabling the measurement of multiple blood constituents, such as oxygen saturation, hemoglobin level, fluid responsiveness, respiration rate, and other physiological parameters that previously required invasive blood sampling and time-consuming laboratory analysis.
According to Dr. Ramsay, Chief of the Department of Anesthesiology and Pain Management at Baylor University Medical Center, "The increasing number of surgical patients with OSA and morbid obesity has increased our need for accurate surveillance. Our study findings showed that Masimo RRa was more accurate, sensitive, and specific to respiration rate changes than capnography. The improved accuracy offered by RRa translates to less false respiratory alarms, earlier detection of respiratory compromise and, ultimately, better patient care."
*To see a summary of all known clinical studies and abstracts on Masimo technologies and noninvasive measurements, please visit: https://professional.masimo.com/evidence/featured-studies/feature/.
1 Ramsay MA, Lagow E, Usman M. Accuracy of Respiration Rate and Detection of Respiratory Pause by Acoustic Respiratory Monitoring in the PACU. NYPGA 2011 Abstract #P-9137; presented December 11, 2011 @ 2 p.m.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®), PVI®, acoustic respiration rate (RRa™), and SEDLine®contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Antelope Valley Hospital Converts to Masimo rainbow SET for State-of-the-Art Noninvasive Patient Monitoring Capabilities
Hospital Standardizes to Pulse CO-Oximetry for Advanced Monitoring of Multiple Physiological Parameters without Invasive Procedures
Irvine, California - November 17, 2011 - Antelope Valley Hospital and Masimo (NASDAQ: MASI) today jointly announce the hospital's conversion to Masimo rainbow® SET Pulse CO-Oximetry™ technology-enabling advanced noninvasive patient monitoring capabilities that offer immediate, pain-free clinical data and physiological information. The hospital-wide conversion equips Antelope Valley's only full-service hospital with the most technologically and clinically-advanced oximetry and noninvasive patient monitoring solutions available-making patient monitoring both pain-less and cost-effective.
"Converting to Masimo rainbow® SET enables Antelope Valley Hospital to provide a higher level of monitoring care that is pain-free and less invasive than traditional capabilities," stated Edward Mirzabegian, CEO of Antelope Valley Hospital. "This new technology allows us to continuously track key blood, fluid, and respiration parameters without using invasive techniques to collect blood. It's like an invisible lifeline that provides our clinicians with immediate, real-time access to advanced clinical intelligence and continuous physiological measurements that were not available 10 years ago. And, because this technology is completely noninvasive, the cost-of-care is reduced to both the hospital and patient-enabling us to deliver an exceptional level of care and safety to our patients cost-effectively."
Masimo rainbow® SET Pulse CO-Oximetry is a breakthrough technology platform that allows hospitals to noninvasively measure and continuously monitor blood constituents and physiological parameters that previously required invasive procedures-including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the 'gold-standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR). The oximetry standard-of-care at leading hospitals worldwide, Masimo rainbow® SET provides immediate real-time results that enable clinicians to more rapidly assess patients and detect and treat adverse, potentially life-threatening conditions earlier.
Antelope Valley Hospital treats more than a quarter of a million inpatients and almost 100,000 emergency patients each year. This conversion ensures that all multiparameter patient monitors, stand-alone oximeters, and sensors feature the advanced noninvasive patient monitoring capabilities of Masimo rainbow® SET-allowing clinicians to easily and painlessly obtain and continuously track vital blood and physiological data for patients in real-time, anywhere in the hospital.
Laura Benesch, Assistant Chief Nursing Officer at Antelope Valley Hospital, said, "We see a lot of trauma patients where early detection and identification of distress is crucial to their care and survival. This is where our conversion to Masimo rainbow SET technology is truly beneficial because it offers us a noninvasive way of continuously assessing patients for key indicators of their physiological health status, like hemoglobin. When a patient is admitted to trauma/ED, OR, or ICU they will have a noninvasive sensor placed on their finger that will monitor their oxygen saturation and hemoglobin levels in real-time, along with seven other vital measurements to help clinicians detect abnormalities that may signal declining health status or a potentially life-threatening condition. This allows us to more tightly monitor our patients' health status to identify and treat problematic conditions earlier, before they become critical or life-threatening."
About Antelope Valley Hospital
Celebrating more than 50 years of caring for the community, Antelope Valley Hospital, a facility of Antelope Valley Healthcare District, is a non-profit, 420-bed hospital that was founded in 1955. The hospital is dedicated to providing quality care and services to everyone in the Antelope Valley. AV Hospital is located at 1600 West Avenue J in Lancaster, California. For further information, please visit www.avhospital.org or call 661-949-5000.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the hospital-wide conversion ensures that all Mater Children's Hospital patients will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo rainbow SET provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient; risks related to our belief that SpHb detects low or falling hemoglobin levels that could be the result of internal bleeding; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Julie Triggs
Antelope Valley Hospital
(661) 949-5530
julie.triggs@avhospital.org
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


Masimo to Present at 23rd Annual Piper Jaffray Health Care Conference
IRVINE, Calif., November 15, 2011 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the 23rd Annual Piper Jaffray Health Care Conference at The New York Palace in New York on Tuesday, November 29, 2011 at 9:30 a.m. Eastern Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


Shands Jacksonville Medical Center Converts to Masimo rainbow SET for State-of-the-Art Noninvasive Patient Monitoring Capabilities
Northeastern Florida Hospital with One of the Biggest and Busiest Trauma Centers Standardizes to Pulse CO-Oximetry for Advanced Monitoring of Multiple Physiological Parameters without Invasive Procedures
Irvine, California – November 10, 2011 – Shands Jacksonville Medical Center and Masimo (NASDAQ: MASI) today jointly announce the conversion of Shands Jacksonville Medical Center to Masimo rainbow®SET Pulse CO-Oximetry™ technology for advanced noninvasive patient monitoring capabilities. The conversion equips the acute care hospital, which also operates one of the biggest and busiest trauma centers, with the most technologically and clinically-advanced pulse oximetry technology—shown to have the best specificity (lowest rate of false alarms) and sensitivity (the highest true alarm detection)—and noninvasive patient monitoring solutions that allow clinicians to measure multiple blood constituents, fluid responsiveness, respiration rate, and other physiological parameters without invasive blood sampling and time-consuming laboratory analysis.1-2
"Converting to Masimo rainbow®SET provides our clinicians with superior patient monitoring capabilities and advanced clinical measurements that offer more immediate access to a higher quality of clinical data at the point-of-care," said Dr. Michael Nussbaum, Chair of the University of Florida College of Medicine Department of Surgery at Shands Jacksonville. "Our evaluations of available pulse oximetry technology led us to the decision to standardize on Masimo's technology platform not only because it works during motion and low perfusion, but because it also provides additional noninvasive blood, fluid and respiration capabilities that other oximeters can't—providing our clinicians with the clinical intelligence they need to deliver an exceptional level of care and safety to our patients."
Masimo rainbow®SET Pulse CO-Oximetry is a breakthrough technology platform that allows hospitals to noninvasively measure and continuously monitor blood constituents and physiological parameters that previously required invasive procedures—including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR). The pulse oximetry standard-of-care at leading hospitals worldwide, Masimo rainbow®SET provides immediate real-time results that enable clinicians to more rapidly assess patients and detect and treat adverse, potentially life-threatening conditions earlier.
Cynthia Gerdik, Director of Clinical Care Nursing at Shands Jacksonville, said the nursing staff relies on Masimo's ability to perform accurately under the most difficult of patient circumstances, "Masimo pulse ox technology is superb—even on patients with cold hands or our littlest pediatric 'wiggle worms', it just works. This was not the case with our prior pulse oximeter brand where it was not uncommon to get no or erroneous measurements on patients with cold extremities, poor perfusion, or even on patients who moved a lot. And, when you rely on pulse ox measurements as a vital sign for severely ill or trauma patients, accurate measurements are a must. Another indicator of Masimo's performance is the confidence our nurses have in its alarms. Today, when an alarm sounds on the Masimo oximeter, our nurses know that it's a real event that they need to immediately check. Masimo has freed us from alarm fatigue due to false alarms. We have trust in Masimo that we didn't have in our prior pulse ox solution."
Together, the University of Florida Health Science Center–Jacksonville and Shands Jacksonville form the region's premier academic health center–UF&Shands, a leader in the education of health professionals, a hub for clinical research and a unique provider of high-quality patient care in Northeast Florida. The conversion ensures that all multiparameter patient monitors, stand-alone oximeters, and sensors feature the advanced noninvasive patient monitoring capabilities of Masimo rainbow®SET—allowing clinicians to easily and painlessly obtain and continuously track vital blood and physiological data for patients in real-time, anywhere in the hospital.
1 Barker SJ. Motion-Resistant Pulse Oximetry: A Comparison of New and Old Models. Anesth & Analg. 2002 Oct;95(4):967-72.
2 Shah N, Estanol L. Comparison of Three New Generation Pulse Oximeters during Motion & Low Perfusion in Volunteers. Anesthesiology 2006;105:A929.
About Shands Jacksonville Medical Center
Shands Jacksonville is a private, not-for-profit hospital affiliated with the University of Florida Health Science Center campuses in Jacksonville and Gainesville. In Jacksonville, UF&Shands employs 5,000 physicians and caregivers and provides care to over 34,000 inpatients and 600,000 outpatients annually. To learn more about Shands Jacksonville, visit www.shands.org
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the hospital-wide conversion ensures that all Shands Jacksonville Hospital patients will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo rainbow SET provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient; risks related to our belief that SpHb detects low or falling hemoglobin levels that could be the result of internal bleeding; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dan Leveton
Shands Jacksonville
(904) 244-3268
daniel.leveton@jax.ufl.edu
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


Masimo Awarded the American Association of Respiratory Cares 2011 Zenith Award
Honored for the Fourth Straight Year at the International Respiratory Congress in Tampa, Florida
Irvine, California November 8, 2011 —Masimo (NASDAQ: MASI) announced today that it has received the American Association of Respiratory Care (AARC) prestigious Zenith Award for the fourth straight year. Honored at the 57th Annual International Respiratory Congress the largest gathering of respiratory care clinicians in the world—the AARC's Zenith Award represents Masimo's unwavering commitment, dedication, and industry leadership in providing best-in-breed, innovative patient care solutions.
AARC Executive Director and CEO, Sam Giordano, stated, "I congratulate Masimo for earning another Zenith Award this year. Companies receiving this prestigious award are chosen based on quality of products, truth in advertising, service, responsiveness, accessibility of sales staff, and support of the respiratory care profession. This year marks the fourth consecutive year that Masimo was voted to receive a Zenith by members of the American Association for Respiratory Care."
The leading national and international professional association for respiratory care, AARC encourages and promotes professional excellence, advances the science and practice of respiratory care, and serves as an advocate for patients and their families, the public, the profession and the respiratory care therapist. As the AARC's top industry honor for quality and service excellence, more than 400 companies were eligible for one of five Zenith Award honors. Award winners are chosen by the association's membership—more than 52,000 respiratory care professionals.
Joe Kiani, Chairman and CEO of Masimo, stated, "Respiratory professionals are a crucial component of the patient care team and their expertise is fundamental to patient recovery. We are proud of our long-standing commitment to the respiratory profession and are honored by the appreciation of so many AARC members who have once again recognized us with the Zenith Award for the superior quality and performance of our products and the service, commitment, and integrity excellence of our Team."
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient carehelping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb), oxygen content (SpOC™), carboxyhemoglobin (SpCO), methemoglobin (SpMet), and Pleth Variability Index (PVI), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa). Masimos rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com. *To see a summary of all known clinical studies and abstracts on Masimo technologies, please visit: https://professional.masimo.com/evidence/featured-studies/feature/.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


Masimo to Present at Lazard Capital Markets Healthcare Conference
IRVINE, Calif., November 8, 2011 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the Lazard Capital Markets Healthcare Conference at The Pierre Hotel in New York on Tuesday, November 15, 2011 at 1:00 p.m. Eastern Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


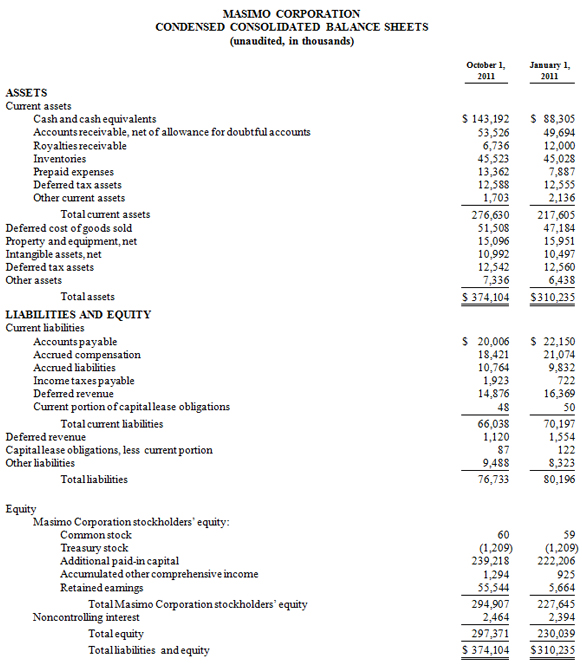
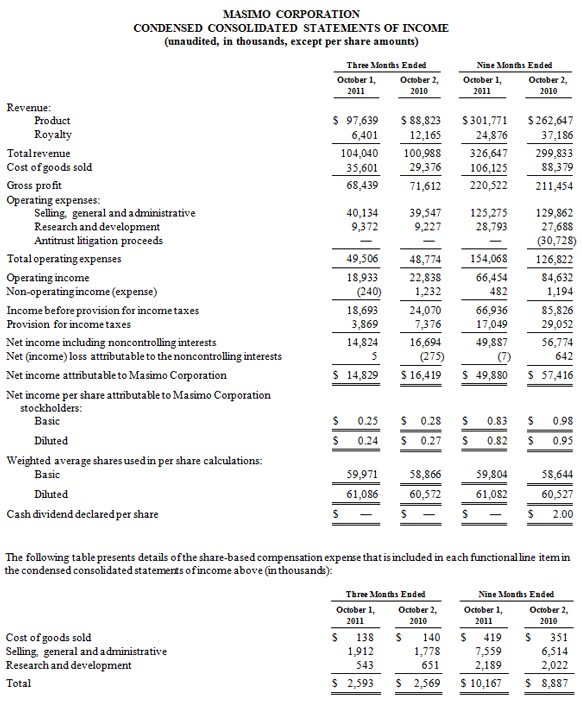
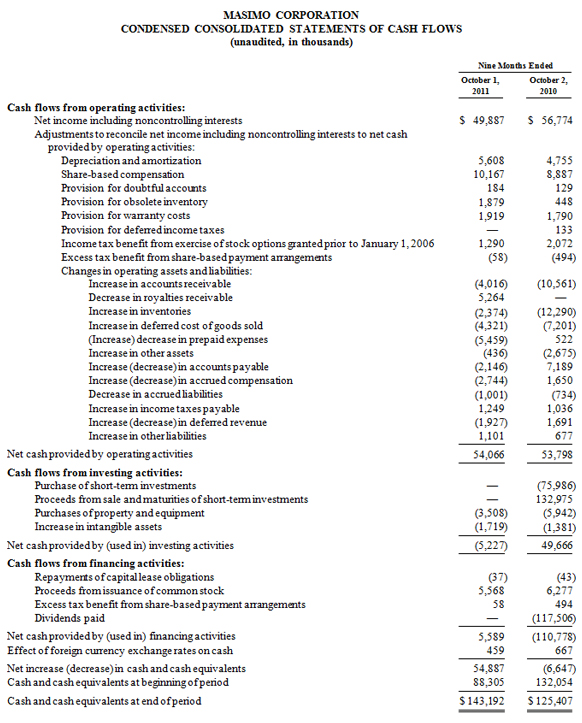
Masimo Reports Third Quarter 2011 Financial Results
Q3 2011 Highlights (compared to Q3 2010):
- Total revenue, including royalties, rose 3% to $104.0 million
- Product revenue rose 10% to $97.6 million
- 33,400 Masimo SET®and Masimo rainbow®SET units were shipped, increasing worldwide installed base by 16%
- Masimo rainbow revenue declined 35% to $7.8 million versus Q3 2010 when the company received an approximately $4 million order from the U.S. Marine Corps
- GAAP EPS was $0.24 versus $0.27 in the prior period, which included $0.01 in one-time expenses
Irvine, California, October 25, 2011 – Masimo (NASDAQ: MASI) today announced its financial results for the third quarter ended October 1, 2011.
Masimo's total third quarter revenue, including royalties, rose 3% to $104.0 million, compared to $101.0 million for 2010s third quarter. The company's third quarter product revenue rose 10% to $97.6 million, compared to $88.8 million for the third quarter of 2010. For the third quarter of 2011, the company's worldwide end-user business grew 18%, while OEM sales were down 20%. Revenue from Masimo rainbow products declined 35% to $7.8 million in the quarter, compared to $11.9 million for the third quarter of 2010 when Masimo received an approximately $4 million rainbow order from the U.S. Marine Corps.
Net income for the third quarter was $14.8 million, or $0.24 per diluted share, compared to reported net income of $16.4 million, or $0.27 per diluted share, in the third quarter of 2010, which included $0.01 per diluted share in one-time expenses.
During the third quarter, the company shipped approximately 33,400 Masimo SET pulse oximetry and Masimo rainbow SET Pulse CO-Oximetry units, excluding handheld units, down 11% compared to approximately 37,500 in the same prior year period. Masimo estimates its worldwide installed base as of October 1, 2011 to be 950,000 units, up 16% from 821,000 units as of October 2, 2010.
As of October 1, 2011, cash and cash equivalents were $143.2 million, compared to $88.3 million as of January 1, 2011.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "Masimo product revenue grew 10% in the period as strong SET performance was partially offset by declines in rainbow and OEM revenue. The 18% rise in our end-user, or direct, business was fueled by strong performance from our U.S. acute care business and even stronger performance from our international business. While we are disappointed with total rainbow revenue in the quarter, we remain confident in the power of rainbow parameters such as SpHb and RAM, which experienced strong year-over-year adhesive sensor unit and revenue growth in the quarter. We expect rainbow to not only help improve the quality and cost of patient care, but to be a catalyst for Masimo's revenue growth over the long term."
Revised Financial Guidance
Masimo now expects fiscal 2011 total revenue to be between $436 million and $439 million, including product revenue between $404 million and $407 million, and royalty revenue between $31.5 million and $32.5 million. Included within the revised 2011 product revenue range is a rainbow revenue expectation of $33 million to $35 million. The company now expects fiscal 2011 GAAP earnings per share to be between $1.04 and $1.06. Each of the components of Masimo's guidance set forth above is an estimate only and actual performance could differ.
Previously, the company's guidance was for fiscal 2011 total revenue to be between $446 million and $463 million, including product revenue at the lower end of the $415 million to $430 million range, and royalty revenue between $31 million and $33 million. Included within the previous product revenue range was a rainbow revenue expectation at the lower end of the $40 million to $50 million range. The company previously expected fiscal 2011 GAAP earnings per share to be at the lower end of the $1.17 and $1.25 range.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 18542610. After the live webcast, the call will be available on Masimo's website through November 25, 2011. In addition, a telephonic replay of the call will be available through November 8, 2011. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 18542610.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care...by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about: our financial condition, results of operations and business generally; our revised expectations for total revenue, royalty revenue and product revenue, including rainbow revenue, and GAAP earnings per share for the full fiscal year 2011; expectations regarding our ability to design and deliver innovative new noninvasive technologies; and global demand for our technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact: Sheree Aronson
Vice President, Investor Relations, Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact: Dana Banks
Manager, Public Relations, Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care...by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Guangdong Biolight Meditech Co., Ltd. Announces the Launch of Its Modular Patient Monitors with Masimo rainbow SET Pulse CO-Oximetry Technology
Irvine, California & Zhuhai, China – October 24, 2011 – Masimo (NASDAQ: MASI) and Biolight, a leading global manufacturer of electronic medical devices and patient monitors, today jointly announced both a worldwide technology licensing agreement and the launch of Masimo rainbow®SET Pulse CO-Oximetry technology in Biolight's new generation of modular patient monitors. The agreement allows Biolight to incorporate the breakthrough noninvasive blood constituent measurement capabilities of Masimo rainbow SET into Biolight's Anyview Series of modular monitors and the Measure-Through Motion and Low Perfusion pulse oximetry capabilities of Masimo SET across Biolight's product line of multiparameter patient monitors—facilitating early detection and treatment of life-threatening conditions.
According to James Yan, Chairman General Manager, Professor Senior Engineering, R&D. "Our corporate mission is to provide innovative medical devices with superior performance and value. The integration of Masimo rainbow SET Pulse CO-Oximetry and Masimo SET Measure-Through Motion and Low Perfusion pulse oximetry technologies is an example of our mission in action. Masimo's innovative technologies and breakthrough noninvasive monitoring solutions provide clinicians with the ability to more rapidly and thoroughly assess a patient's physiological status and make better clinical and treatment decisions."
Integrating Masimo rainbow SET Pulse CO-Oximetry technology into Biolight's Anyview Series of modular monitors will provide immediate and continuous access to additional clinical measurement data—enabling clinicians to detect and treat the early signs of hemodynamic instability, internal bleeding, and respiratory distress sooner. Masimo rainbow SET Pulse CO-Oximetry is a breakthrough technology platform revolutionizing patient monitoring by significantly expanding the ability to noninvasively measure and continuously track multiple blood constituents that previously required intermittent, invasive procedures, including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and perfusion index (PI), in addition to the 'gold standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), and pulse rate (PR).
"The inclusion of Masimo technologies will allow us to optimize the design and manufacture of state-of-the-art patient monitors and provide our domestic and international customers with access to the latest in cutting-edge, noninvasive physiological measurements," continued Yan. "As one of the first medical device manufacturers in China to incorporate Masimo's rainbow SET technology, we expect our combined product offering will allow clinicians to advance the delivery of healthcare across all patient care settings."
Incorporating Masimo's core Signal Extraction Technology (SET) pulse Oximetry technology into Biolight's non-modular "M" and "V" Series of patient monitors will also help clinicians more accurately measure a patient's oxygenation during challenging conditions such as patient motion and low perfusion to facilitate better treatment decisions – as clinically proven in over 100 independent studies. Utilizing patented signal processing technologies, including parallel engines and adaptive filters, Masimo SET delivers accurate and reliable measurements of a patient's true oxygenation status when conventional pulse oximetry technologies don't — reducing false alarms by over 95% (sensitivity) and expanding true alarm detection to over 97% (specificity).1
Rick Fishel, President of Worldwide OEM Business and Corporate Development at Masimo, stated, "Biolight is one of the first patient monitoring manufacturers in China to integrate Masimo rainbow SET Pulse CO-Oximetry technology. This "first-to-market" commitment in Biolight's modular Anyview series of patient monitors provides Biolight customers with advanced physiological monitoring across a broad range of monitors which offer a unique combination of upgradeability and point-of-care flexibility."
1 Shah N., Estanol L. "An Evaluation of Three New Generation Pulse Oximeters during Motion & Low Perfusion in Volunteers." Anesthesiology 2006; 102: S-75.
To see a summary of all known clinical studies and abstracts on Masimo technologies, please visit: https://professional.masimo.com/evidence/featured-studies/feature/.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
About Guangdong Biolight Meditech Co., Ltd.
Guangdong Biolight Meditech Co., Ltd. is a high-tech company located in Zhuhai, focusing on developing, producing and marketing medical instruments including patient monitoring systems, central monitoring systems, and electrocardiograph and digital colposcope systems. Founded in 1993, Biolight has successfully developed advanced medical electronic equipment in nearly 10 series and 40 types, including multi parameter patient monitors, central monitoring stations, and digital colposcope imaging systems. Biolight products have a considerable market share in China. We are also selling well in the international market and we have distributors in Asia, the Middle East, Europe and America. In 2003, Biolight built a new technology industrial park which covers 20,000sqm for developing and producing patient monitoring systems.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that the superior performance of Masimo SET pulse oximetry and the clinical importance of the upgradeable Masimo rainbow®SET Pulse CO-Oximetry™ technology platform have contributed to the growth and adoption of Masimo technologies, risks related to our belief that Masimo technologies help hospitals to improve patient care and enhance patient safety initiatives, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
Phone: (949) 297-7348
Email: dbanks@masimo.com
Thomas Lee
Biolight Co., Ltd.
Phone: 0086-756-3399996
Email: thomas@blt.com.cn
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.

New Clinical Studies Presented at the American Society of Anesthesiologists Annual Meeting Show Benefits of Masimo Noninvasive Technologies: SpHb, PVI, RRa, and SEDLine
Irvine, California – October 19, 2011 – Masimo (NASDAQ: MASI) announced today that over 25 new clinical studies evaluating Masimo noninvasive patient monitoring technologies were presented at the largest gathering of anesthesiologists in the world, the American Society of Anesthesiologists (ASA) Annual Meeting in Chicago, Illinois. The following studies highlight the positive clinical outcomes and patient safety impact of Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®), pleth variability index (PVI®), acoustic respiration rate (RRa™), and SEDLine®brain function monitoring.
SpHb®& PVI®
Researchers Steven Frank, M.D., James Rothschild, M.D., and John Ulatowski, M.D., at Johns Hopkins Hospital in Maryland found that the combination of continuous SpHb and PVI monitoring "may improve the efficacy and safety of the intraoperative autologous normovolemic hemodilution (IAHD) blood conservation technique that helps avoid allogeneic blood transfusions." The study, conducted in patients undergoing major abdominal or orthopedic surgery, concluded that SpHb was advantageous in "eliminating the delay in intraoperative decision-making that occurs when Hb measurements are obtained by conventional laboratory testing" while PVI was a "useful index of intravascular volume during the significant fluid shifts that occurred with IAHD, with increasing PVI indicating hypovolemia and decreasing PVI indicating adequate fluid resuscitation and blood re-infusion." 1
Two studies, conducted by the Long Beach Veterans Healthcare System in California, found that noninvasive SpHb measurements provided valuable clinical assessment information in remote, rural, and community settings. Nitin Shah, M.D., and Kinjal Shah, B.S., led a study using the Radical-7 in rural India, which concluded that SpHb measurements provided "reliable information compared to the invasive method" with a bias and precision of 0.2+/- 1.2 g/dL and was "very convenient to use in a rural camp setting" while also eliminating "biohazard risks of venipuncture and blood handling."2 The second study, led by Nitian Shah with Deval Modi, M.B, B.S., performed at two community health fairs in Long Beach concluded that the Pronto-7 spot-check device, with a bias and standard deviation of -0.06 +1.07 g/dL, provides "similar values and offers acceptable accuracy" when compared to values obtained from laboratory analysis of invasive blood samples. Researchers also noted the advantages of Pronto-7 in that it "gives immediate result" and has the potential to be "very helpful in spot checking for anemia in the general community without the need for exhaustive set-up or processing time."3
Two separate studies showed that the new In Vivo Adjustment™ feature for noninvasive SpHb measurements, included as part of the new 2011 Radical-7, along with the newest generation rainbow ReSposable Sensors (Rev. E) helped clinicians to improve the agreement in subsequent comparisons between invasive (tHb) and noninvasive (SpHb) hemoglobin measurements. Researchers Kathleen Richard, M.D., Timothy Quill, M.D., Thomas Dodds, M.D., and Matthew Koff, M.D., M.S., at Dartmouth Hitchcock Medical Center in New Hampshire found that the "accuracy and descriptive statistics improved when each time-matched SpHb value was adjusted by the magnitude of difference between the first tHb and corresponding SpHb." Researchers concluded that "if this type of adjustment were performed in real-time in vivo (intra-operatively), overall accuracy of SpHb values may be further improved," which may also "add value to care" during high risk surgical procedures in patients with co-morbidities and "in many other clinical locations and scenarios (ICU, PACU, pediatrics, emergency medicine, surgical ward)."4 Another study, conducted by Ryo Miyashita, M.D., Shigekazu Sugino, M.D., Yukitoshi Niiyama, M.D., Mitsuko Mimura, M.D., Ph.D., and Michiakii Yamakage, M.D., Ph.D., in Japan at the Sapporo Medical University School of Medicine, found that "SpHb values corrected using the novel program were more accurate than those obtained conventionally." Study results showed that In Vivo Adjustment improved bias and standard deviation from 1.1 + 1.0 to 0.0 + 0.61 g/dL) and concluded that In Vivo Adjustment should "contribute to the accurate measurement of SpHb in the clinical setting."5
Researchers Peter Winch, M.D., Aymen Naguib, M.D., Julie Rice, R.N., and Joseph Tobias, M.D., at Nationwide Children's Hospital showed that SpHb measurements obtained in pediatric patients undergoing acute blood loss correlated to hemoglobin values obtained from a point-of-care device. Researchers evaluated the use of SpHb in a pediatric population undergoing phlebotomy and concluded that SpHb provided "accurate and continual real-time data which can inform medical care".6
In a separate study, also conducted at Nationwide Children's Hospital, the same research team found that monitoring changes in PVI could be used as a guide for volume replacement during isovolemic hemodilution in pediatric patients undergoing congenital cardiac surgery. Patients were evaluated in two groups—group 1 had starting PVI values less than 14 and group 2 had starting PVI values greater than 14. Results showed that the average crystalloid replacement in group 1 was 5ml/kg, while volume replacement in group 2 was 11ml/kg in order to maintain the same hemodynamics during hemodilution. Researchers noted that the "data demonstrates the possible advantage of using the PVI value as a tool for identifying patients who would be good candidates for isovolemic hemodilution."7
Rainbow Acoustic Monitoring™ for RRa™
Researchers Basavana Goudra, M.D., and Lakshmi Penugonda, M.D., at the Hospital of the University of Pennsylvania, evaluated RRa measurements obtained using the Masimo Rad-87 during upper gastrointestinal endoscopy and found it had the "best accuracy and precision (-0.3 +/- 1.0 bpm) of monitoring respiration rate," whereas EtCO2 (-0.6 +/- 6.1 bpm) and impedance pneumography (0.2 +/- 4.3 bpm) are "subject to frequent false alarms." Results showed that EtCO2 had the "highest incidence of false alarms" with 45 false alarms out of 52 events, while RRa had the "lowest rate of false alarms" with just 3 out of 52 events.8
SEDLine®
In a study conducted at Loma Linda University Medical Center, researchers Kevin Nasseri-Noori, M.D., Deborah McIvor, M.D., Frank Hsu, M.D., Moses Olson, B.S., Martin Allard, M.D., Travis Losey, M.D., Mark Macknet, M.D., and Richard Applegate, M.D., demonstrated that the SEDLine 4-channel (PSA array) brain function monitor "correctly detected an excessively deep intraoperative burst suppression therapy (IBST) pattern given a nearly isoelectric EEG" in 16 out of 19 events during intracranial surgery. With an overall correlation of 93% between SEDLine (PSA array) and EEG—showing strong agreement—study results indicate that SEDLine may be an effective tool for IBST detection versus standard EEG, which is expensive and requires a special technician in the room.9
According to Nitin Shah, MD, Chief of Surgical ICU at Long Beach VA Hospital and Professor of Anesthesiology at Loma Linda University, "Noninvasive SpHb is the way of the future. It is truly a blessing for a developing country with limited resources. Patients appreciate that it eliminates the need for traditional needle stick blood draws, so they are much more relaxed and willing to complete the pain-free SpHb testing. And, for healthcare professionals it is unbelievable. There are no biohazard risks, no formal training required to operate, and no calibration required with SpHb testing and they get instant results that they can use immediately to advise their patients right on the spot. In my view, SpHb should be routinely used in clinics and community settings across the globe."
Basavana Goudra, MD, Assistant Professor of Anesthesiology and Critical Care at the Hospital of the University of Pennsylvania, commented, "Masimo rainbow acoustic monitoring is better than anything else we have at the moment for accurate and reliable respiration rate measurements. Not only was it easy to use in the majority of the patients enrolled in our study, but our results show that it is the perfect solution for routine use in upper GI endoscopy procedures and post-operative monitoring as EtCO2 is not useful in these situations.
1 Frank S, Rothschild J, Ulatowski J. "Continuous Noninvasive Hemoglobin Monitoring for Jehovah's Witness Patients Undergoing Intraoperative Autologous Normovolemic Hemodilution" ASA 2011 Presentation A408.
2 Shah N, Shah K. "Evaluation of a Pulse CO-Oximeter for Noninvasive Hemoglobin Measurement in Adult Population in Rural India" ASA 2011 Presentation A283.
3 Shah N, Modi D. "Accuracy of Noninvasive Hemoglobin Measurement Through a Pulse CO-Oximeter Compared to Venous Blood Draw in a Community Setting" ASA 2011 Presentation A285.
4 Richard K, Quill T, Dodds T, Koff M. "Improved Accuracy and Trending of Noninvasive Hemoglobin Measurements with 'In Vivo Adjustment'" ASA 2011 Presentation A1667.
5 Miyashita R, Sugino S, Niiyama Y, Mimura M, Yamakage M. "Improved Noninvasive Total Hemoglobin Measurements After In Vivo Adjustment" ASA 2011 Presentation A410.
6 Winch P, Naguib A, Rice J, Tobias J. "The Accuracy of Noninvasive Hemoglobin Monitoring Following Phlebotomy in a Pediatric Patient Population" ASA 2011 Presentation A411.
7 Naguib A, Winch P, Rice J, Tobias J. "Can the Starting Pulse Oximeter Derived Pleth Variability Index (PVI) Predict Total Crystalloid Replacement During Isovolemic Hemodilution in Congenital Cardiac Surgery?" ASA 2011 Presentation A207.
8 Goudra B., Penugonda L. "Monitoring Respiration in Upper GI Endoscopy Anesthesia" ASA 2011 Presentation A246.
9 Nasseri-Noori K, McIvor D, Hsu F, Olson M, Allard M, Losey T, Macknet M, Applegate R. "Prospective Comparison of Global Electroencephalogram to Frontal SEDLine Electroencephalogram Monitoring for the Evaluation of Intraoperative Burst Suppression During Elective Intracranial Surgery" ASA 2011 Presentation A499.
*In-Vivo Adjustment is pending FDA 510(k) clearance.
**To see a summary of all known clinical studies and abstracts on Masimo technologies and noninvasive measurements, please visit: https://professional.masimo.com/evidence/featured-studies/feature/.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com .
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®), PVI®, acoustic respiration rate (RRa™), and SEDLine®contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo to Report Third Quarter 2011 Financial Results after Market Close on October 25, 2011
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., October 13, 2011 -- Masimo (NASDAQ: MASI) announced today that it will release third quarter financial results for the period ended October 1, 2011, after the market closes on October 25, 2011. The conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 18542610. After the live webcast, the call will be available on Masimo's website through November 25, 2011. In addition, a telephonic replay of the call will be available through November 8, 2011. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 18542610.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact: Media Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Launches the 2011 Radical-7 with rainbow® Acoustic Monitoring and In Vivo Adjustment™ 2011 Radical-7 will be Shown October 15-19 at the 2011 American Society of Anesthesiologists (ASA) Annual Meeting in Chicago
Irvine, California – October 13, 2011 – Masimo (NASDAQ: MASI) announced today FDA 510(k) clearance and CE Mark of its new Radical-7®with rainbow®Acoustic Monitoring capability for continuous display of the acoustic respiration rate (RRa™) waveform and measurements. Also new to the Radical-7 is an In Vivo Adjustment™ (pending FDA 510(k) clearance in the U.S.) that allows clinicians to, for the first time, adjust the noninvasive measurements to the specific patient and laboratory reference device they use for invasive blood testing.
rainbow®Acoustic Monitoring
The new Radical-7 provides clinicians with the option to measure RRa and display the acoustic waveform directly on the monitor screen with the pleth waveform overlayed—allowing them to observe changes in breathing upon inhalation and exhalation. With this new display capability, clinicians may be able to more readily detect respiratory pause events where there is an absence of breathing, high ambient noise that can degrade the acoustic signal, and improper sensor placement.
 |
Respiration rate is defined as the frequency of breathing expressed as the number of breaths per minute and is considered a critical vital sign in assessing the physiological status of hospitalized patients; however, current methods for respiration rate monitoring are limited by reliability or patient tolerance. In contrast, Masimo rainbow Acoustic Monitoring is completely noninvasive and virtually unnoticeable to the patient—featuring an innovative adhesive sensor with an integrated acoustic transducer that is easily and comfortably applied to the patient's neck to detect upper airway acoustic vibrations on the surface of the skin during the respiratory cycle. Using patented acoustic signal processing that leverages Masimo's revolutionary Signal Extraction Technology (SET), the respiratory signal is separated and processed to display continuous RRa measurements.
In Vivo Adjustment Allows Users to Account for Individual Patient Bias from Laboratory Reference Device
The measurement of blood components such as oxygen saturation, methemoglobin, carboxyhemoglobin, and total hemoglobin have inherent and expected variability within and between noninvasive and invasive measurement techniques and from patient to patient. For example, all of the noninvasive measurements use a technique coined 'patient calibration' which takes the noninvasive monitor's detected light that is transmitted or reflected from the patient at different values and matches them to empirically gathered invasive blood measurements from a small number of volunteers and in some cases patients. However, individuals may vary from the pool of patients used in the 'patient calibration' and hence provide a bias for the particular patient. In addition, many clinicians are unaware that laboratory reference devices that measure oxygen saturation or hemoglobin can have great variability from each other. In fact, hemoglobin can vary 2 g/dL or more from one manufacturer's product to another. And, physiologic factors such as the blood source (venous or arterial), site and time of blood draws, blood draw technique, and patient body position are recognized in the clinical literature to add variability to hemoglobin levels. As a result, noninvasive and invasive measurements can vary in the same patient depending on the methods and invasive equipment used.
The In Vivo Adjustment feature available in the new Radical-7 now provides the option to adjust the noninvasive oxygen saturation (SpO2), total hemoglobin (SpHb®), carboxyhemoglobin (SpCO®), and methemoglobin (SpMet®) values displayed on the monitor to the laboratory reference value used at the site by patient. With this new feature, clinicians can adjust the noninvasive value at the beginning of a monitoring period to account for individual patient variation and the laboratory reference value and continuously trend the noninvasive value to the patient and reference laboratory value until the next blood sample and laboratory analysis.
"With the addition of the new In Vivo Adjustment feature, Masimo refines its solution for three major limitations that have always plagued pulse oximetry: 1) the inability to measure-through motion and low perfusion (virtually eliminated by Masimo SET); 2) the inaccuracy of SpO2 in the presence of dyshemoglobins (they can now be monitored with SpCO and SpMet); and 3) the inability of one calibration curve to fit all patients (an individual patient's SpO2 can be adjusted to a reference arterial blood gas measurement)," stated Steven Barker, PhD, MD, Chairman of Masimo's Scientific Advisory Board.
Juan Soliveres, M.D., University Hospital in Valencia, Spain, stated, "There are many invasive devices that measure Hb and the results they report may be different from one device to the next. Even laboratory co-oximeters will report different Hb values when the same blood sample is run through different machines of the same make and model. When comparing invasive Hb measurements with noninvasive SpHb measurements, an In Vivo Adjustment feature—to remove measurement bias and calibrate SpHb measurement relative to the lab device—is an important enhancement that should contribute to improved Hb correlations between these measurements."
In addition, the new Radical-7 now features a larger number display with pulse oximetry measurements that are 40% larger than before to improve visibility. And for added safety, the device now includes an optional Alarms All Mute feature that allows the customer to enable or disable the ability for users to mute (silence) alarms on the monitor and adds a layer of protection that password-protects this feature—enabling only qualified clinicians to turn-on the Alarms All Mute feature. This feature safeguards against indiscriminate and accidental silencing of monitor alarms that can place patients at increased risk of unrecognized distress and deterioration, while enabling qualified clinicians (through password access) the ability to focus their attention on immediate patient care, interventions, and intensive care resuscitations in sensitive care situations without the audible distractions of monitor alarms.
Masimo Founder and CEO, Joe Kiani, stated, "With the latest Radical-7, we have once again expanded what clinicians can expect from a pulse oximeter; this time by adding the ability to monitor comfortably and reliably a patient's respiration rate. And, with In-Vivo Adjustment, clinicians can, when they need to, get very accurate measurements that are not only patient focused, but adjusted to their laboratory reference devices."
According to Michael O'Reilly, MD, Chief Medical Officer at Masimo, "On hospital general care floors around the world, the problems associated with aggressive post-operative pain management with patient-controlled analgesia (PCA) pumps, rising patient co-morbidities, and decreasing nurse-to-patient ratios have led to dramatic increases in adverse events and avoidable patients deaths. As a result, leading patient safety organizations have been calling for continuous monitoring of oxygenation and ventilation on post-surgical general care floor patients. Today, thanks to Masimo innovations, the need for a reliable SpO2 and respiration rate monitor for patients on general care floors is finally realized—and patients can be safer than ever before."
*The new Radical-7 Pulse CO-Oximeter has FDA 510(k) and CE Mark clearances for rainbow®Acoustic Monitoring and CE Mark clearance for In-Vivo Adjustment. In-Vivo Adjustment is pending FDA 510(k) clearance.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com .
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that the new Radical-7 offers clinicians expanded functionality and new features that improve patient monitoring; risks related to our belief that the new RRa waveform overlayed with the pleth waveform will allow clinicians to more readily detect respiratory pause events where there is an absence of breathing, high ambient noise that can degrade the acoustic signal, and improper sensor placement; and risks related to our belief that the new In Vivo Adjustment feature will enable clinicians to calibrate noninvasive measurements directly to their specific invasive blood sampling reference device for improved device-to-device comparisons; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo Rainbow Acoustic Monitoring Technology Cleared for Market in Japan Acoustic Respiration Rate (RRa) Technology Now Available for First Time in Japan in the Masimo Rad-87 Pulse CO-Oximeter
Tokyo, Japan and Irvine, California – October 10, 2011 – Masimo (NASDAQ: MASI) announced today that it has received Japanese Ministry of Health Labor & Welfare (MHLW) regulatory clearance for its Rad-87®Pulse CO-Oximeter with rainbow®Acoustic Monitoring technology—providing noninvasive and continuous acoustic respiration rate (RRa™) measurements that are accurate, easy-to-use, and enhance patient compliance. Available for the first time in Japan, RRa represents a breakthrough solution for measuring respiration rate in real-time to track and trend patient breathing—offering automated assessments that may enable earlier detection of respiratory compromise and patient distress.
Respiration rate is defined as the frequency of breathing expressed as the number of breaths per minute and is considered a critical vital sign in assessing the physiological status of hospitalized patients. Continuous monitoring of respiration rate is especially important for post-surgical patients receiving patient-controlled analgesia (PCA) for pain management as sedation can induce respiratory depression and place patients at considerable risk of serious injury or death.1-4 Although the Anesthesia Patient Safety Foundation (APSF) recommends continuous oxygenation and ventilation monitoring in all patients receiving opioids,5-6 current methods for respiration rate monitoring are limited by reliability or patient tolerance.
Dr. Toshiyasu Suzuki, MD, PhD, Professor of Anesthesiology, Tokai University School of Medicine in Kanagawa Japan, who is one of the first Rad-87 with RRa users, stated, "The number of surgeries has been increasing at acute care hospitals since DPC started in Japan. As a result, the number of postoperative patients sent to the general ward has increased and nurses have more workload, especially at the night shift. We have concerns about patient safety. Anesthesiologists tend to administer postoperative patients muscle relaxant and/or pain relief drugs such as Morphine, Fentanyl, Remifentanil. Those patients are often at risk of respiratory failure when excessive amounts of these drugs are administered. The respiratory monitoring for those patients is currently done by pulse oximeter and/or a visual observation, however that is not enough. Now we have a solution. I strongly recommend Rad-87 with RRa: it is a simple use and the RRa accuracy is as good as ETCO2, on top of that SpO2 can be monitored at the same time. I am assured that Rad 87 with RRa will be a powerful tool for clinicians and nurses on a postoperative patient risk management."
Michael Ramsay, M.D., Chief of the Department of Anesthesiology and Pain Management at Baylor University Medical Center in Dallas, Texas, stated, "Breathing adequately is what matters most. Masimo acoustic respiration rate (RRa) provides clinicians with the ability to automatically and continuously monitor the breathing status of post-surgical patients in general care or post-anesthesia settings—alerting them to the first sign of an abnormal or compromised breathing pattern that may be indicative of airway obstruction or respiratory distress. And, the Masimo Acoustic Sensor is virtually unnoticeable when worn—making it extremely patient-friendly."
Masimo rainbow Acoustic Monitoring features an innovative adhesive sensor with an integrated acoustic transducer that is easily and comfortably applied to the patient's neck to detect upper airway acoustic vibrations on the surface of the skin during the respiratory cycle. Using patented acoustic signal processing that leverages Masimo's revolutionary Signal Extraction Technology (SET), the respiratory signal is separated and processed to display continuous RRa measurements.
Masimo Founder and CEO, Joe Kiani, stated, "Solving 'unsolvable' problems that have plagued patient monitoring is key to advancing medicine and improving patient care. Masimo Rainbow Acoustic Monitoring addresses a longstanding and growing need to accurately and easily monitor patient breathing, which is expected to improve patient safety and decrease the cost of care in hospitals. It also adds another compelling reason for hospitals to choose the Masimo rainbow SET upgradeable platform for pulse oximetry, Pulse CO-Oximetry, and now Acoustic Monitoring."
1 Joint Commission on Accreditation of Healthcare Organizations. Sentinel Event Alert: Patient controlled analgesia by proxy. Chicago: JCAHO, 2004
2 Institute for Safe Medication Practice. Safety issues with patient-controlled analgesia: Part 1-How errors occur. Huntingdon Valley: ISMP, 2003
3 Institute for Safe Medication Practices. Safety issues with patient-controlled analgesia: Part II - How to prevent errors Huntingdon: ISMP, 2003
4 Bird M. Acute pain management: a new area of liability for anesthesiologists ASA Newsletter. Park Ridge: American Society of Anesthesiologists, 2007
5 Weinger MB. Dangers of Postoperative Opioids: APSF Workshop and White paper address prevention of postoperative respiratory complications. APSF Newsletter, 2006;21:61-7. http://www.apsf.org/newsletters/html/2007/winter/winter2006-7.htm
6 Stoelting RK, Weinger MB. Dangers of Postoperative Opioids -- Is There a Cure? APSF Newsletter, 2009;24:25-26. http://www.apsf.org/newsletters/html/2009/summer/index.htm
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo rainbow acoustic monitoring technology and RRa respiration rate measurements provide accurate, reliable, real-time data for all patients under all conditions and offer automated assessments, which may enable earlier detection of respiratory compromise and patient distress, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium


Masimo Achieves New Milestone-Ships One Millionth Oximeter
Milestone Event Celebrates Wellness and Commitment to Advancing the Standard-of-Care
Irvine, California - September 29, 2011 - Masimo (NASDAQ: MASI) announced today that it shipped its 1,000,000th Masimo SET®pulse oximeter and rainbow®SET Pulse CO-Oximeter (excluding hand-held units). The one millionth oximeter went to Sisters of Charity Hospital in Buffalo, New York, as part of a new NICU, Labor & Delivery and Nursery technology conversion. To celebrate this milestone, Masimo held a Millionth Oximeter Celebration at its corporate headquarters in Irvine, California today.
Joe Kiani, Founder, chairman and CEO of Masimo, stated "We are excited to achieve this milestone and share the moment with the Sisters of Charity Hospital and our local community. As the recipient of our millionth Masimo rainbow SET oximeter, Sisters of Charity Hospital joins an ever-growing list of premier hospitals that have chosen Masimo
to provide the new standard-of-care in patient monitoring. And, celebrating our millionth oximeter is a reminder of how our technologies have advanced patient care and improved patient safety for millions."
Founded with the goal of solving the 'unsolvable' problems plaguing patient care, Masimo innovations have revolutionized pulse oximetry and noninvasive blood constituent, hemodynamics, and respiration monitoring. When conventional pulse oximetry technologies failed to measure accurately and reliably during patient motion and low perfusion and other companies could not solve the problem, Masimo succeeded with the invention of Masimo SET®Measure-Through Motion and Low Perfusion pulse oximetry technology. When conventional pulse oximetry failed to measure accurately in the presence of dyshemoglobins (like carboxyhemoglobin and methemoglobin) and other pulse oximetry technologies could not isolate and measure dyshemoglobins, Masimo succeeded again. With Masimo rainbow®SET Pulse CO-Oximetry™ clinicians can now continuously measure and monitor multiple blood constituents, dyshemoglobins, and physiological parameters that previously required invasive procedures. And, thanks to Masimo Patient SafetyNet™, continuous patient monitoring on general care floors-where 80% of the hospital population is cared for-is now finally a reality at hospitals worldwide.
"When we started Masimo, our mission was to 'improve patient outcome and reduce cost of care by taking noninvasive monitoring to new sites and applications.' While we feel like we have just begun, I am proud that we have already accomplished every aspect of our mission," continued Joe Kiani.
Today, Masimo medical breakthroughs and technology innovations allow more patients to be accurately and reliably monitored and are transforming lives in ways that were not possible with conventional pulse oximetry. Spanning every care area and clinical setting, Masimo solutions are helping clinicians to improve patient safety and clinical outcomes by dramatically increasing the early detection of physiological abnormalities and life-threatening conditions, reducing retinopathy of prematurity (ROP), enabling the screening of congenital heart defects (CHD) in newborns, and decreasing risky blood transfusions in the operating room, as well as rescue events and costly ICU transfers on general care floors are just some of the ways Masimo is making a measureable difference for patients around the world.
According to Antonio Garcia at Frost & Sullivan, "Masimo simply takes the guess work out of pulse oximetry, so clinicians never have to guess what brand is the most reliable. Masimo's novel product portfolio has added a new dimension to noninvasive patient monitoring equipment that has considerably facilitated early detection of challenging patient conditions."
The superior performance and innovative capabilities of Masimo SET and rainbow SET technologies and products have produced more than 12 medical firsts, garnered over 60 industry awards, and fueled the growing adoption of Masimo at hospitals around the world. In just four years, Masimo has doubled its 2007 milestone of 500,000 oximeters shipped on the heels of its 200,000th oximeter milestone, achieved in 2004. And, today more than half of the top hospitals listed on the U.S. News & World Report Best Hospitals Honor Roll have converted to Masimo technology.
Masimo SET has been clinically proven in over 100 independent studies to provide the most accurate and reliable SpO2 and pulse rate measurements, even under the most challenging conditions of patient motion and low peripheral perfusion. Utilizing patented signal processing technologies, including parallel engines and adaptive filters, Masimo SET delivers accurate and reliable measurements of a patient's true oxygenation status when conventional pulse oximetry technologies don't reducing false patient monitor alarms by over 95% (sensitivity) and expanding true alarm detection to over 97% (specificity).1 Before Masimo SET, pulse oximetry was a fair weather friend-useful only under ideal conditions, but unreliable when accurate monitoring was needed most. With Masimo SET, pulse oximetry is now not only a foul weather friend, but is being used in places and care areas it couldn't before. As a result, the industry has not only come to depend on continuous noninvasive physiologic monitoring by Masimo SET pulse oximetry, but has made it the leading Measure-Through Motion and Low Perfusion SpO2 solution incorporated into over 100 multiparameter monitors and 50 monitoring brands.
In addition, Masimo has taken noninvasive monitoring to new applications by bringing new noninvasive capabilities and solutions to the point-of-care that eliminate the pain, complexity, and delays associated with invasive blood draws and procedures. Masimo rainbow SET®Pulse CO-Oximetry™, a breakthrough technology platform featuring the accuracy and reliability of Masimo SET, is continuing to revolutionize patient monitoring by significantly expanding the ability to noninvasively capture, continuously track and monitor multiple blood constituents that previously required invasive procedures, including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), perfusion index (PI), and acoustic respiration rate (RRa™), in addition to the 'gold standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), and pulse rate (PR).
Over the Years, Leading Clinicians Have Recognized Masimo Innovations as Medical Breakthroughs
In 2002, Dr. Charles Durbin, Department of Anesthesiology, at the University of Virginia Health System in Charlottesville, Virginia, warned clinicians of the impact of conventional pulse oximetry on patient care and safety, stating that, "Monitors providing false alarms distract caregivers from other tasks and require attention to trouble shoot or fix the monitor. This decreases efficiency, increases costs, and increases the likelihood that monitoring will not be continued because of caregiver distrust of the device. Ultimately, patient safety may be adversely affected. This is particularly important because oximetry is being used outside the ICU on the general ward where the effects of false alarms are even more likely to negatively affect patient outcome. Recent studies on human error and patient safety point to caregiver cognitive overload and distraction (termed latent conditions) as one cause of patient injury or error." In contrast to conventional oximetry, Dr. Durbin's research studies concluded that "Masimo SET pulse oximetry technology was more reliable and accurate than conventional pulse oximetry, has been shown to perform well in poor perfusion states and during motion, resulted in far less monitoring failure time than conventional oximetry, and presented more reliable oximetry data to clinicians, which resulted in 68% faster weaning of FI02 with 34% fewer arterial blood gas draws (ABGs)."2
In 2003, Dr. Augusta Sola, lead researcher and author of a breakthrough protocol to reduce ROP using Masimo SET pulse oximetry stated, "I tell my friends, my family, and my neighbors in America and overseas, a good thing happened in 1989-Masimo SET technology changed the way we can use pulse oximetry." Studies conducted at Cedars-Sinai Medical Center in Los Angeles showed that Masimo SET helped to reduce the rate of ROP from 12% to 2.5% and the need for ROP laser treatment from 4.5% to 0%.3
In 2005, Dr. Steven Barker, Head of the Department of Anesthesiology at the University of Arizona College of Medicine, commented that, "In the 25-year history of pulse oximetry, no one has produced an instrument that will function accurately on patients suffering from carbon monoxide poisoning. The development of a pulse oximeter that can separately measure carboxyhemoglobin and oxyhemoglobin will be a major advance in healthcare…and would soon become a standard of care in emergency rooms, ambulances, and many other critical care settings."4
Numerous lifesaving accounts have highlighted the impact of noninvasive carboxyhemoglobin (SpCO) measurements from Masimo's handheld Rad-57, including one from Wake County EMS Chief, Skip Kirkwood, M.S., J.D., EMT-P, who said, "We believe that all 50+ hotel guests might have been dead at dawn if it were not for this lifesaving intervention from Masimo."
Masimo's First Customers are Still Ardent Supporters and Users of the Technology Almost 20 Years Later
One of Masimo's first installations was at Hannover Medical School in Hannover, Germany, where Prof. Christian F. Poets, M.D. was the Director of the Neonatal Intensive Care Unit (NICU) at the time. Now the Medical Director for the Department of Neonatology at Tubingen University Hospital (Germany), Dr. Poets stated, "In my view, Masimo has revolutionized the continuous, noninvasive measurement of oxygenation by making it much more motion-resistant and capable of producing reliable measurements at low perfusion. This has been made possible by excellent engineering and their willingness to listen to their customers' needs."
Dr. Katsuyuki Miyasaka, Director of the Perioperative Center at St. Luke's International Hospital in Tokyo, Japan, is another one of Masimo's very first customers, responsible for the first hospital-wide installation of Masimo SET pulse oximeters at the National Center for Child Health and Development (Tokyo). "When I first experienced using a Masimo SET pulse oximeter on a hyperkinetic infant in 1993, I was amazed and assured that Masimo SET would be the first-ever pulse oximeter to solve the problem of patient motion artifact and low perfusion. Congratulations to Masimo for shipping one million pulse oximeters. I am sure that SpHb and other emerging noninvasive innovations enabled by Masimo rainbow SET technology will further contribute to improved patient care and save even more patients' lives."
Peter U. Bergmann, FACHE, President and CEO of Sisters of Charity Hospital and one of Masimo's newest customers, stated, "We are honored to be the recipient hospital of Masimo's millionth oximeter. Converting the NICU, labor & delivery and nursery departments to Masimo SET provides us with the most advanced oximetry technology enabling the most advanced care for newborns. At Sisters of Charity Hospital, you can be confident that your baby is receiving the most advanced medical and nursing care available using state-of-the-art technology with a family-centered approach to care. Our number one priority is to ensure that you and your baby receive the best care possible during your stay with us and Masimo oximeters are an important part of monitoring our tiniest patients."
1 Shah N., Estanol L. "An Evaluation of Three New Generation Pulse Oximeters during Motion & Low Perfusion in Volunteers." Anesthesiology 2006; 102: S-75.
2 Durbin C.G. Jr., Rostow S.K. "More Reliable Oximetry Reduces the Frequency of Arterial Blood Gas Analyses and Hastens Oxygen Weaning after Cardiac Surgery: A Prospective, Randomized Trial of the Clinical Impact of a New Technology." Crit Care Med. 2002 Aug;30(8):1735-40.
3 Chow L.C., Wright K.W., Sola A.; CSMC Oxygen Administration Study Group. "Can Changes in Clinical Practice Decrease the Incidence of Severe Retinopathy of Prematurity in Very Low Birth Weight Infants?" Pediatrics. 2003 Feb;111(2):339-45.
4 Barker S.J., Curry J., Redford D., Morgan S. "Measurement of Carboxyhemoglobin and Methemoglobin by Pulse CO-Oximetry: A Human Volunteer Study." Anesthesiology. 2006 Nov;105(5):892-7.
To see a summary of all known clinical studies and abstracts on Masimo technologies, please visit: https://professional.masimo.com/evidence/featured-studies/feature/.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
About Sisters of Charity Hospital
Sisters of Charity Hospital was Buffalo's first hospital, with a tradition of superior care dating back to 1848. Today, the hospital is part of the Catholic Health system that provides care to Western New Yorkers across a network of hospitals, primary care centers, imaging centers, and several other community ministries. With 290 licensed beds, Sisters of Charity Hospital provides comprehensive medical care and treatment to more than 15,000 inpatients, 33,000 emergency room visitors, and 560,000 outpatients each year.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that the superior performance of Masimo SET pulse oximetry and the clinical importance of the upgradeable Masimo rainbow®SET Pulse CO-Oximetry™ technology platform have contributed to the growth and adoption of Masimo technologies, risks related to our belief that Masimo technologies help hospitals to improve patient care and enhance patient safety initiatives , as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Published Study Demonstrates the Absolute and Trend Accuracy of Masimo Noninvasive and Continuous Total Hemoglobin (SpHb) Monitoring inIntensive Care Unit Patients
Researchers Cite Masimo SpHb as a "Significant Expansion of Existing Monitoring Technology"
Irvine, California - September 27, 2011 -Masimo (NASDAQ: MASI) announced today that findings from a new study published in the Journal of Critical Care Medicine demonstrate that noninvasive and continuous hemoglobin (SpHb) monitoring has absolute and trending accuracy similar to widely used invasive methods of hemoglobin measurement with additional advantages. Study highlights that "the ability to measure hemoglobin noninvasively and continuously with SpHb has significant potential to facilitate hemoglobin monitoring, hasten the detection of acute anemia, and avoid the complications, expense, and discomfort associated with invasive blood draws."1
Total hemoglobin measurement is one of the most frequently ordered laboratory tests. But, traditional blood analysis has many drawbacks, including complexity, time-consuming turnaround times that can impact clinical decisions, and blood loss due to invasive blood draws that have been found to contribute substantially to the anemic conditions that commonly occur in critically ill patients. Other studies suggest that blood loss from invasive blood draws induce iatrogenic anemia, with over 90% of intensive care unit (ICU) patients becoming anemic by the third day of ICU admission.2 In contrast, Masimo SpHb offers a completely noninvasive method of continuously measuring hemoglobin levels without removing a drop of blood. This study is the first to evaluate the performance and accuracy of SpHb against three invasive techniques commonly used in the ICU for measuring hemoglobin.
In the study, conducted at the University Hospital in Poitiers, France, researchers compared SpHb measurements (obtained noninvasively using Radical-7 version 7.6.0.1 and Adult rainbow ReSposable Sensors R2-25, Rev. E) to invasive laboratory measurements analyzed by a satellite lab CO-Oximeter (Siemens RapidPoint 405), a HemoCue point-of-care device, and a laboratory hematology analyzer-considered the reference device (Sysmex XT-2000i) in 62 ICU patients. Results showed bias and limits of agreement for SpHb were 0.0 +1.0 g/dL, 0.3 + 1.3 g/dL for HemoCue, and 0.9 + 0.6 g/dL for the satellite lab CO-Oximeter compared to the reference device. SpHb showed similar trend accuracy to the satellite lab CO-Oximeter, whereas the HemoCue point-of-care device did not appear to follow the trend of the laboratory analyzer or reference device. As a result of these findings, researchers concluded that SpHb "has absolute accuracy and trending accuracy similar to widely used invasive methods of hemoglobin measurement at bedside" and has "the additional advantages of providing continuous measurements, noninvasively, which may facilitate hemoglobin monitoring in the intensive care unit."
The researchers commented that although invasive CO-Oximeters are an accepted laboratory standard for assessing hemoglobin and have high interdevice reproducibility, SpHb "had the best accuracy as evidenced by the highest concordance co-efficient correlation and the lowest Arms."
SpHb is available as part of Masimo rainbow SET Pulse CO-Oximetry-a breakthrough technology platform that noninvasively and continuously measures multiple blood constituents and physiological parameters that previously required invasive procedures, including:
total hemoglobin (SpHb), oxygen content (SpOC), carboxyhemoglobin (SpCO), methemoglobin (SpMet), Pleth Variability Index (PVI), perfusion index (PI), and acoustic respiration rate (RRa), in addition to the 'gold standard' Measure-Through Motion and Low Perfusion performance of Masimo SET oxyhemoglobin (SpO2), and pulse rate (PR).
To see a summary of all known clinical studies and abstracts on Masimo SpHb, please visit:
https://professional.masimo.com/evidence/featured-studies/feature/.
1 Frasca D, Dahyot-Fizelier D, Catherine K, Levrat Q, Debaene B, Mimoz O. "Accuracy of a Continuous Noninvasive Hemoglobin Monitor in Intensive Care Unit Patients." Crit Care Med October 2011; Vol. 39, No. 10. For further reading, visit: http://journals.lww.com/ccmjournal/Abstract/2011/10000/Accuracy_of_a_continuous_noninvasive_hemoglobin.10.asp
2 Rodriguez RM, Corwin HL, Gettinger A, et al. "Nutritional Deficiencies and Blunted Erythropoietin Response as Causes of the Anemia of Critical Illness." J Crit Care 2001; 16:36-41. For further reading, visit: http://burndoc.net/nutrition_epogen.pdf
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb), oxygen content (SpOCTM), carboxyhemoglobin (SpCO), methemoglobin (SpMet), and Pleth Variability Index (PVI), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic MonitoringTM, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications" Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SpHb provides continuous monitoring of hemoglobin levels for all patients, and risks related to our assumptions that Masimo SpHb monitoring has the potential to decrease the frequency of invasive blood testing, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation.
The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


U.S. Health & Human Services Makes Critical Congenital Heart Defect Screening Using Motion-Tolerant Pulse Oximetry a Nationwide Newborn Screening Standard Implementation Strategy and Protocols Recommended by Federal Advisory Committee of Leading Associations (AAP, ACC, AHA, HHS) Along With Findings From New UK Study Using Masimo SET Support Motion-Tolerant, Low Perfusion Pulse Oximetry For Improved CHD Screening in Newborns
Irvine, California – September 23, 2011 – Masimo (NASDAQ: MASI) announced today that the U.S. Department of Health & Human Services (HHS) has added Critical Congenital Heart Defects (CCHD) screening using motion-tolerant pulse oximetry as a national newborn screening standard. In addition, the largest UK study of pulse oximetry screening for CHD detection, published online in the Lancet (appearing in the November 2011 print edition), demonstrates that when Masimo SET®Measure-Through Motion and Low Perfusion pulse oximetry was used to screen newborns before hospital discharge, it enabled clinicians to increase CHD detection and provided a cost-effective method for universal screening with high sensitivity.1
An International Problem—Undetected / Undiagnosed CHD in Newborns
Among the approximately 4.2 million babies born in the U.S. annually,2 CHD is the most prevalent form of birth defect and is the #1 cause of infant death.3 HHS estimates that approximately 7-9 babies per 1,000 live births have some form of CHD.3 Yet, up to 30% of infant deaths from CHD occurred before diagnosis4—leading many to question the effectiveness of current newborn screening methods. Current methods of CHD detection rely largely on newborn physical examination but fail to identify approximately 50% of CHD cases5—leaving many vulnerable newborns undiagnosed at hospital discharge.
The Strategy for a Nationwide Solution—"Motion-Tolerant Pulse Oximetry"
In a letter dated September 21, 2011, HHS Secretary Kathleen Sebelius outlined the decision to adopt expert panel recommendations for universal CCHD screening by pulse oximetry for all newborns into federal Recommended Uniform Screening Panel (RUSP) Guidelines—the national newborn screening system standards and policies. Citing the "emerging evidence base for the utility of early diagnosis and detection of CCHD via measurement of blood oxygen saturation" and the "momentum and commitment" at the state and federal levels," Sebelius directed federal agencies to "proceed expeditiously with implementation." The federal Agency Plan of Action outlined by HHS calls for the National Institutes of Health (NIH) to fund technology, process, care, and outcomes research activities; the Centers for Disease Control and Prevention (CDC) to fund surveillance activities to monitor infant morbidity and mortality outcomes; and the Health Resources and Services Administration (HRSA) to guide development of screening standards and implementation infrastructure, including training materials.3
In August 2011, a panel of pediatric and cardiac experts from the American Academy of Pediatrics (AAP), the American College of Cardiology (ACC), and the American Heart Association (AHA), in conjunction with the HHS Secretary's Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC), acted on the HHS 2010 recommendation6 and outlined a strategy for routine screening of newborns to improve detection of CHD.7 The 28-page report recommends that newborn screening be done with "motion-tolerant pulse oximeters that report functional oxygen saturation, have been validated in low perfusion conditions, have been cleared by the FDA for use in newborns, and have a 2% root-mean-square accuracy." The report also outlined a 5-point implementation strategy and follow-up procedures, which includes screening, diagnostic confirmation, electronic results reporting, primary care follow-up, surveillance and tracking.
Gerard R. Martin, M.D., F.A.A.P., F.A.C.C., Senior Vice President of the Center for Heart, Lung and Kidney Disease at Children's National Medical Center in Washington, D.C., stated, "The excellent results that we can now achieve in correcting critical congenital heart defects make timely diagnosis even more important. Adding pulse oximetry screening as an important adjunct to fetal ultrasound and newborn physical examination will help to ensure that no baby is missed. This is major win for babies born with congenital heart diseases, as well as the families and providers who care for them."
Latest Published Study to Show Masimo SET Improves Early CHD Detection in Newborns
In the current Lancet-published study, researchers screened 20,055 asymptomatic newborn babies (gestation >34 weeks) at six UK maternity units using Masimo SET pulse oximetry before discharge. Newborns with oxygen saturation (SpO2) of less than 95% in either limb or a difference of more than 2% between limbs were considered positive for CHD and underwent echocardiography. Results showed that 53 babies had major CHDs (24 critical)—a prevalence of 2.6 per 1,000 live births. Masimo SET pulse oximetry achieved sensitivity of 75% (95% CI 53·29–90·23) for critical CHDs (causing death or requiring invasive intervention before 28 days) and 49% (95% CI 35·06–63·16) for all major CHDs (causing death or requiring invasive intervention within 12 months of age.)1
Findings also showed that Masimo SET pulse oximetry helped clinicians increase CHD detection by 34% versus antenatal ultrasonography alone (from 35 cases to 53 cases). In addition, out of the 0.8% false-positive results (169), 27% had other problems that required medical intervention (6 were significant but not major congenital heart defects and 40 were other illnesses like respiratory disorders and infections)—showing that the sensitivity of Masimo SET enabled identification of other life-threatening newborn conditions that likely would have otherwise gone undiagnosed. Researchers concluded that: "Pulse oximetry is a safe, feasible test that adds value to existing screening. It identifies cases of critical congenital heart defects that go undetected with antenatal ultrasonography. The early detection of other diseases is an additional advantage."
According to Dr. Mitchell Goldstein, Neonatologist and Associate Professor of Pediatrics at Loma Linda University Children's Hospital in California, stated, "Congenital heart defects are difficult to detect because many newborns do not show outward signs of failure until they decompensate. Today, congenital heart disease screening by Masimo SET pulse oximetry represents a safe and inexpensive way to screen for heart disease before the baby has a life-threatening event. Federal recommendations for universal screening underscore the critical importance of what SET has done to make pulse oximetry a clinically useful tool."
Other Studies Prove: Pulse Oximetry Technology Matters
In addition to the current Lancet-published study, two of the largest CHD studies conducted to date have also shown that Masimo SET is effective in screening newborns for CHD, including a Swedish study of 39,821 newborns published in the British Medical Journal8 and a Norwegian study of 50,008 newborns published in the Journal of Pediatrics.9
Masimo SET (Signal Extraction Technology) is clinically proven in over 100 independent and objective studies to provide the most accurate and reliable SpO2 and pulse rate measurements, even under the most challenging conditions of patient motion and low peripheral perfusion. Utilizing patented signal processing technologies—including parallel engines and adaptive filters—to deliver accurate and reliable measurements of a patient's true oxygenation status, Masimo SET has been shown to reduce false patient monitor alarms by over 95% and expand true alarm detection to over 97%. Because of this superior performance, Masimo SET is the leading Measure-Through Motion and Low Perfusion SpO2 solution incorporated into over 100 multiparameter monitors and 50 monitoring brands—including Atom, Datascope, GE Medical, Medtronic, Philips, Spacelabs, Welch Allyn, and Zoll, among others.
Joe Kiani, Founder, Chairman, and CEO of Masimo, commented that, "We commend HHS, the federal advisory committee, individual states, and the countless medical professional, public health leaders, and advocates that worked so hard to give our nation's smallest and most vulnerable patients a resounding voice. Adding CHD screening by Measure-Through Motion and Low Perfusion pulse oximetry is a significant advancement of our nation's newborn screening standard that will save untold newborn lives and help to prevent the agony of families who are left to cope with the devastating effects of undetected CHD."
To date, because of the overwhelming clinical evidence and appeals by grassroots advocates made up largely of parents with babies affected by CHD, Maryland and New Jersey have passed legislation promoting CHD screening by pulse oximetry in newborns with similar screening initiatives taking place in Beijing, China and the U.K. For a complete list of states' status on CHD screening legislation, visit: www.cchdscreeningmap.com
* Note: Masimo SET technology is not indicated to diagnose or treat congenital heart defects.
Editor's note:
References:
1. Andrew K Ewer, Lee J Middleton, Alexandra T Furmston, Abhay Bhoyar, Jane P Daniels, Shakila Thangaratinam, Jonathan J Deeks, Khalid S Khan. "Pulse oximetry screening for congenital heart defects in newborn infants (PulseOx): a test accuracy study." The Lancet 2011: Vol. 378; No. 9793; pp 785-794. For further reading visit: http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(11)60753-8/fulltext#article_upsell.
2. Sutton, PD. "Recent trends in births and fertility rates through June 2010." National Center for Health Statistics Health E-Stat 2010. Available at: http://www.cdc.gov/nchs/data/hestat/births2010/births2010.htm
3. Secretary of Health & Human Services' letter to the Secretary's Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC); dated September 21, 2011. For further reading visit: http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/recommendations/correspondence/cyanoticheartsecre09212011.pdf.
4. De-Wahl Granelli A, Mellander M, Sunnegardh J, Sandberg K, Östman -Smith I. "Screening for duct-dependent congenital heart disease with pulse oximetry: a critical evaluation of strategies to maximize sensitivity." Acta Paediatr 2005;94:1590-6. For further reading visit: http://onlinelibrary.wiley.com/doi/10.1111/j.1651-2227.2005.tb01834.x/abstract;jsessionid=E0F922B8E4E2950354BB77D36463ED96.d01t03?systemMessage=Wiley+Online+Library+will+be+disrupted+3+Sep+from+10-12+BST+for+monthly+maintenance.
5. Wren C, Richmond S, Donaldson L. "Presentation of congenital heart disease in infancy: implications for routine examination." Arch Dis Child Fetal Neonatal Ed. 1999;80:F49–F53. For further reading visit: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1720871/.
6. Secretary's Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC); Recommendation Letter to HHS Secretary Kathleen Sebelius; October 15, 2010. For further reading visit: http://www.hrsa.gov/heritabledisorderscommittee/correspondence/HowellLettertoSebeliusOct152010.pdf. Secretary of Health and Human Services; Letter to SACHDNC on CHD Screening Recommendation; April 20, 2011. For further reading visit: http://www.hrsa.gov/heritabledisorderscommittee/correspondence/CCCHDSecResponse042011.pdf.
7. Alex R. Kemper, William T. Mahle, Gerard R. Martin, W. Carl Cooley, Praveen Kumar, W. Robert Morrow, Kellie Kelm, Gail D. Pearson, Jill Glidewell, Scott D. Grosse, R. Rodney Howell. "Strategies for Implementing Screening for Critical Congenital Heart Disease." Pediatrics; published online ahead of print Aug. 22, 2011. For further reading visit: http://pediatrics.aappublications.org/site/misc/2011-1317.preprint.pdf.
8. Anne de-Wahl Granelli, Margareta Wennergren, Kenneth Sandberg, Mats Mellander, Carina Bejlum, Leif Inganäs, Monica Eriksson, Niklas Segerdahl, Annelie Ågren, Britt-Marie Ekman-Joelsson, Jan Sunnegårdh, Mario Verdicchio, Ingegerd Östman-Smith. "Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39 821 newborns." British Medical Journal (BMJ) January 2009; 338:a3037. For further reading visit: http://www.bmj.com/content/338/bmj.a3037.full.
9. Meberg A, Brugmann-Pieper S., Due R. et al. "First Day of Life Pulse Oximetry Screening to Detect Congenital Heart Defects." Journal of Pediatrics 2008: 761-765. For further reading visit: http://www.ncbi.nlm.nih.gov/pubmed/18492511.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo SET improves CHD detection in newborns before hospital discharge, risks related to our assumptions of the repeatability of clinical results obtained, and risks related to our assumptions that Masimo SET pulse oximetry technology is a superior solution for CHD detection and newborn screening applications, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.

Centre Hospitalier de Chaumont in France Implements State-of-the-Art
Remote Monitoring System to Advance Patient Safety
Masimo Patient SafetyNet™ Provides a New Level of Safety and
Peace of Mind for Hospitalized Patients
Paris, France & Irvine, California – September 12, 2011 – Patients admitted to Centre Hospitalier (CH) de Chaumont in France can feel safer thanks to a new technological guardian angel. Today, in addition to hospital staff, patients at Chaumont are being safeguarded by Masimo Patient SafetyNet™—a new, advanced remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable patient deaths and injuries associated with failure to rescue events—one of today's most common medical errors.1
"Patients need to know that there are systems dedicated specifically to safeguarding their health and safety in place at some hospitals, and we're on the forefront of this effort," stated Vartan Iknoyan, Biomedical Chief Engineer at Centre Hospitalier de Chaumont. "As a Patient SafetyNet™ hospital, Chaumont now utilizes this state-of-the-art remote noninvasive monitoring system to help keep our patients from deteriorating to the point of critical danger—helping us to achieve better outcomes, recoveries, and patient satisfaction in the process."
Developed by Masimo (NASDAQ: MASI), Patient SafetyNet™ helps keep patients safer by noninvasively, continuously measuring and tracking the underlying physiological condition of up to 80 patients on four floors to detect real-time changes and abnormalities that signal declining health status. The moment a patient's condition deteriorates, the system automatically sends wireless alerts directly to the pager or smartphone of assigned clinicians—prompting an immediate, potentially lifesaving response at the patient's bedside. Patient SafetyNet™ has been clinically-proven to significantly decrease traumatic critical events by 65% and costly ICU transfers by 48% to improve patient outcomes and reduce costs.1
According to Dr. Bernard Simon, Head of the Department of Pneumology and Oncology at Centre Hospitalier de Chaumont, Patient SafetyNet has made a big impact on care. "First thing we noticed was a significant reduction in false alarms because the technology is more advanced and accurate. Not having to chase down false alarms has allowed our clinicians to be more efficient and focus more attention on the care and treatment of patients. The system's ability to monitor and detect real-time changes in a patient's condition enable us to anticipate serious problems and intervene earlier, which allows us to reduce critical events, transfers to the ICU, and length of stays by improving clinical decision-making so that our patients can get better sooner."
The Patient SafetyNet™ system features a noninvasive patient sensor [finger or acoustic respiration neck sensor] and bedside patient monitor [Masimo Rad-87®or Radical-7®Pulse CO-Oximeter™] that continuously captures vital physiological measurements—including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), acoustic respiration rate (RRa™), oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR)—and wirelessly transmits them to the
central monitoring station, which displays the real-time clinical status of all connected patients and instantly routes alarms to the pager or smartphone of assigned clinicians. (See Figure 1 for the Complete System)1 Taenzer AH, Pyke JB, McGrath SP, Blike GT. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study" Anesthesiology; February 2010; Vol 112, Issue 2, pp 282-287.
About Chaumont Hospital
The origin of the original hospital goes back to the beginning of this millenium, but closed its doors in 1748 for maladjustment and insalubrity. Donations allowed the startup, in 1767, of the hospital of Chaumont. Under the material and spiritual control of the Brotherhood of the Girls of Charity, the hospital increased in size during the 19th century, was destroyed and then replaced by part of the new technical wing sheltering the laboratory (Villemin Pavilion), the urgent care reception and the SAMU/SMUR. Then right before the First World War, the MAILLOT and LARREY wings were established and reserved for the hospitalization of soldiers.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic MonitoringTM, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo Patient SafetyNet can help keep patients safer by noninvasively, continuously measuring and tracking their underlying physiological condition to help hospitals avoid preventable patient deaths and injuries associated with failure to rescue events, risks related to our assumptions of the repeatability of clinical results obtained, and risks related to the system's ability to significantly decrease traumatic critical events and costly ICU transfers to help improve patient outcomes and reduce costs, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Dana Banks
Masimo Corporation
Director of Corporate Communications
+ (949) 297-7348
dbanks@masimo.com
Audrey Valckenaere
Centre Hospitalier de Chaumont
(33) 03 25 30 71 49
Audrey.valckenaere@ch-chaumont.fr
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Shriners Hospitals for Children®- Honolulu: First in Hawaii to Implement Masimo Noninvasive Hemoglobin Monitoring Technology
Technology Reliably Monitors Hemoglobin Levels without Needles Masimo Noninvasive and Continuous Hemoglobin (SpHb®) Helps Hospital Reduce the Risk of Undetected Bleeding and Unnecessary Blood Transfusions to Improve Patient Safety
 Honolulu, Hawaii & Irvine, California – August 31, 2011 – The healthcare professionals at Shriners Hospitals for Children®- Honolulu, which perform hundreds of orthopaedic surgeries on children each year, are using the latest in medical technology from Masimo (NASDAQ: MASI) to noninvasively monitor patient blood levels during selected surgeries. Without the use of needles, the innovative technology instantly lets the healthcare team know if a patient has internal bleeding so they can initiate a lifesaving blood transfusion the moment one is needed. Masimo noninvasive and continuous hemoglobin (SpHb®)monitoring technology is also helping the nonprofit Honolulu hospital save precious time and money associated with lab results and unnecessary blood transfusions.
Honolulu, Hawaii & Irvine, California – August 31, 2011 – The healthcare professionals at Shriners Hospitals for Children®- Honolulu, which perform hundreds of orthopaedic surgeries on children each year, are using the latest in medical technology from Masimo (NASDAQ: MASI) to noninvasively monitor patient blood levels during selected surgeries. Without the use of needles, the innovative technology instantly lets the healthcare team know if a patient has internal bleeding so they can initiate a lifesaving blood transfusion the moment one is needed. Masimo noninvasive and continuous hemoglobin (SpHb®)monitoring technology is also helping the nonprofit Honolulu hospital save precious time and money associated with lab results and unnecessary blood transfusions.
"Knowing whether a patient's hemoglobin blood level is improving or falling to a critical point where a blood transfusion is necessary provides a good outcome for our young patients," said Harlan Klein, MD, Chief of Anesthesiology at Shriners Hospitals for Children®- Honolulu, who monitors vital signs of patients during surgeries.
According to National Institutes of Health (NIH) estimates, about five million patients receive blood transfusions each year in the United States, and more than halfof heart surgery patients need at least one transfusion of red blood cells.1 Blood transfusions, which replenish blood lost during surgeries, can be lifesaving, but they can also cause life-threatening complications. Blood transfusions can improve outcomes only when used in the right patient, at the right time, and in the right dose. Masimo SpHb allows healthcare professionals to accurately and reliably track hemoglobin changes occurring in real-time—providing earlier indications of directional changes than intermittent invasive hemoglobin values.2
|
Figure 1—Masimo Radical-7 |
"In the past, we've only received glimpses of our patients' hemoglobin levels from lab measurements, but now we have complete and real-time hemoglobin visibility," said Richard DiBucci, RN, Inpatient and Surgical Services Manager of the Honolulu hospital. "We not only can spot hemoglobin changes as they occur, but also can see where they are heading. This helps us to identify upward and downward hemoglobin trends on a second-by-second basis, which has been of tremendous value."
Masimo SpHb is part of Masimo rainbow®SET Pulse CO-Oximetry—a breakthrough technology platform that allows hospitals to noninvasively measure and continuously monitor blood constituents and physiological parameters that previously required invasive procedures—including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the 'gold-standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR). The oximetry standard-of-care at leading hospitals worldwide, Masimo rainbow®SET provides immediate real-time results that enable clinicians to more rapidly assess patients and detect and treat adverse, potentially life-threatening conditions earlier.
1 National Institutes of Health; NIH News; June 21, 2010; www.public.nhlbi.nih.gov/newsroom/home/GetPressRelease.aspx?id=2710
2 L. Lamhaut, R. Apriotesei, M. Lejay, B. Vivien, P. Carli. "Comparison Between a New Noninvasive Continuous Technology of Spectrophotometry-based and RBC Count for Haemoglobin Monitoring During Surgery with Hemorrhagic Risk" Eur J Anaesthesiol 2010; 27 (Suppl 47): 3AP7-1.
About Shriners Hospitals for Children®- Honolulu
Shriners Hospitals for Children®– Honolulu provides innovative pediatric orthopaedic care to children in Hawaii and throughout the Pacific Basin. There are two qualifications for care at Shriners Hospital: Children must be under age 18 and have an orthopaedic condition that can be treated. All care at the hospital is provided to a child, regardless of the patient's family ability to pay. More than 28,000 children have received orthopaedic care at the Honolulu hospital since it opened in 1923. There are 22 Shriners Hospitals in the United States, Canada and Mexico providing advanced care for children with orthopaedic conditions, burns, spinal cord injuries, and cleft lip and palate.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Nathan Hokama
Strategic Communication Solutions
(808) 226-7470
Dana Banks
Masimo Corporation
(949) 297-7348
(949) 309-7759 (mobile)
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Pronto-7™ Receives Japanese and Canadian Regulatory Clearances
Irvine, California – August 29, 2011 – Masimo (NASDAQ: MASI) today announced the Japanese Ministry of Health Labor & Welfare (MHLW) and Health Canada regulatory clearances of Masimo Pronto-7™— enabling clinicians throughout Japan and Canada to quickly and conveniently measure total hemoglobin (SpHb®), SpO2, perfusion index, and pulse rate without removing a drop of blood.
Hemoglobin is one of the most commonly ordered tests in both the hospital and pre-hospital settings because it is critical to assessing blood loss following trauma, during surgery, and during hospital stay, as well as a patient's need for a blood transfusion. However, traditional lab testing requires a painful needle stick for the patient, time-consuming blood draws for the clinician, and typically provides delayed results. Masimo SpHb provides immediate real-time hemoglobin results that enable clinicians to more rapidly assess patients and detect and treat internal bleeding and low hemoglobin conditions earlier. And, although Masimo noninvasive and continuous total hemoglobin (SpHb) monitoring—commercially available in both Japan and Canada for over a year—is the preferred technology for hospitals and inpatient care centers, access to quick and easy spot-check hemoglobin measurements are valuable for a variety of healthcare assessment applications, including physician offices, outpatient care centers, and pre-hospital emergency settings.
Pronto-7 Makes Noninvasive Hemoglobin Testing a Reality in Japan and Canada Japanese and Canadian regulatory clearances for the palm-sized, handheld Pronto-7 and noninvasive finger sensors make it possible for clinicians to take the pain and wait out of traditional hemoglobin blood testing. The Pronto-7 offers a breakthrough solution for measuring total hemoglobin, SpO2, perfusion index, and pulse rate in less than one minute without the needles, time-consuming laboratory analysis, risk of blood contamination, hazardous medical waste, and patient discomfort associated with traditional blood tests. With dimensions of just 13 cm x 7.2 cm x 2.5 cm (5.1" x 2.8" x 1") and weight of 296 grams (10.5 ounces), the palm-sized Pronto-7 puts the power of noninvasive hemoglobin spot-check testing, along with SpO2, perfusion index, and pulse rate into any clinician's hands in various clinical settings—including physician offices, hospitals, clinics, and on the scene of medical emergencies.
Japanese and Canadian regulatory clearances for the palm-sized, handheld Pronto-7 and noninvasive finger sensors make it possible for clinicians to take the pain and wait out of traditional hemoglobin blood testing. The Pronto-7 offers a breakthrough solution for measuring total hemoglobin, SpO2, perfusion index, and pulse rate in less than one minute without the needles, time-consuming laboratory analysis, risk of blood contamination, hazardous medical waste, and patient discomfort associated with traditional blood tests. With dimensions of just 13 cm x 7.2 cm x 2.5 cm (5.1" x 2.8" x 1") and weight of 296 grams (10.5 ounces), the palm-sized Pronto-7 puts the power of noninvasive hemoglobin spot-check testing, along with SpO2, perfusion index, and pulse rate into any clinician's hands in various clinical settings—including physician offices, hospitals, clinics, and on the scene of medical emergencies.
In addition to Normal Mode, Pronto-7 offers Max Sensitivity Mode that allows measurement over a broader range of patients and three noninvasive sensor sizes to accommodate the range of finger diameters. Each sensor is color-coded to make size identification easy (Small-Yellow, Medium-Red, and Large-White) and optimized to improve performance in low ambient temperatures. And, because of the device's embedded 802.11 b/g and Bluetooth communication capability, wireless printing or emailing of test results is enabled with future upgrades that will allow for wireless transmission to electronic health record (EHR) systems.


Prior to the introduction of the Pronto-7, Masimo received U.S. FDA 510(k) and CE Mark clearance and first introduced the ability to noninvasively and continuously measure total hemoglobin (SpHb) in 2008 using its Radical-7 bedside Pulse CO-Oximeter. In 2009, Masimo launched Pronto®—its first handheld noninvasive spot-check device for total hemoglobin, SpO2, perfusion index, and pulse rate measurements. And in 2010, Masimo established Medicare reimbursement for transcutaneous hemoglobin measurement in the United States.
Masimo Founder and CEO, Joe Kiani, stated: "The potential impact of noninvasive total hemoglobin, SpO2, perfusion index, and pulse rate spot-check measurement offers healthcare providers in Japan and Canada the opportunity to transform the way and amount of time it takes to deliver the right care, at the right time to patients. We believe that the Pronto-7 will help clinicians to better assess more patients and make better clinical decisions based on the availability of quick and noninvasive hemoglobin measurement."
* Masimo Pronto-7 is pending FDA 510(k) clearance in the U.S.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the enhanced Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at Morgan Stanley Global Healthcare Conference
IRVINE, Calif., August 29, 2011 — Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the Morgan Stanley Global Healthcare Conference at the Grand Hyatt New York on Tuesday, September 13, 2011, at 1:35 p.m. Eastern Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Obtains CE Mark for SEDLine, Launches EEG-Based Brain Function Monitors in European Market
European Availability Expands Access to Critical Care Brain Function Platform
Irvine, California – August 24, 2011 – Masimo (NASDAQ: MASI) announced today that it has received CE Mark for its SEDLine brain function monitor—expanding access to and availability of SEDLine's advanced neuromonitoring technology to hospitals and clinicians across Europe. The only brain function monitor with four separate EEG channels in a single integrated algorithm SEDLine represents a critical advancement for the practice of anesthesia in Europe—more complete data for a more complete picture of brain function and sedation.
SEDLine expands the scope of real-time data and improves the management of an anesthetic case by enabling more individualized titration. Four channels of high-quality EEG data provide information about both sides of the brain to facilitate immediate detection of asymmetrical activity and yields a single sophisticated algorithm to give accurate, reliable information about a patient's response to anesthesia. Masimo's SEDLine Patient State Index (PSI™), a calculated measure of brain activity that reflects the patient's current level of sedation/anesthesia1, provides additional data to enhance anesthetic control and facilitate rapid assessment. With demonstrated reliability under challenging clinical conditions and superior resistance to cautery2, SEDLine offers a cost-effective solution for brain function monitoring that helps clinicians achieve targeted sedation throughout all phases of anesthesia—in the OR and ICU.
 Caption (top): SEDLine's 4-channels of high-quality EEG data provide information on both sides of the brain.Caption (bottom): Four active leads in the SEDLine sensor collect a higher volume of data in key areas of the frontal lobe. |
|---|
According to Michael Ramsay, M.D., Chief of Anesthesiology and Pain Management & President of the Research Institute at Baylor University Medical Center in Dallas, Texas, "For many of the liver transplants and cardiac anesthesia cases I participate in, SEDLine provides me with real-time access to more comprehensive brain activity data, which is especially critical when a surgical patient's hemodynamic parameters are rapidly changing. Because of SEDLine's unique four separate EEG channels in a single integrated algorithm—enabling greater control and sedation management—I believe it will also complement the use of Target Control Infusion narcotic based anesthesia, which is a widely used technique in Europe."
The introduction of EEG-based monitoring systems such as SEDLine to provide guided titration has been shown to reduce the amount of anesthetic used and decrease recovery time when used as an adjunct to current standard clinical practice.3-4 In fact, these studies show that SEDLine PSI facilitated more individualized titration of anesthesia that led to patients receiving significantly less anesthetic and recovering an average of 67% faster—ultimately enabling faster OR to PACU throughput with patients meeting discharge eligibility19% faster than non-SEDLine-monitored patients.
Masimo Founder and CEO, Joe Kiani, stated: "The risks associated with the expanded use of short-acting anesthetic drugs like Propofol, where patients go in and out of the sedation state very quickly, makes it increasingly important for anesthesiologists to maintain tighter control. Masimo's SEDLine PSI and our popular 4-channel waveforms help anesthesiologists keep their patients where they want them to be—improving sedation control and the management of anesthetized patients."
SEDLine is currently used by top U.S. hospitals, including Baylor Medical Center in Dallas, TX; Massachusetts General Hospital in Boston, MA; Stanford University Medical Center in Palo Alto, CA; New York Columbia Presbyterian Hospital in NYC; Loma Linda University Medical Center in Loma Linda, CA.
References:
1. Prichep LS, Gugino LD, John ER, et al. The Patient State Index as an indicator of the level of hypnosis under general anesthesia. Br J Anaesth. 2004,92:393-399. Available online at http://bja.oxfordjournals.org/cgi/content/full/92/3/393.
2. White PF, Tang J, Ma H, Wender RH, Sloninsky A, Kariger R. Is the Patient State Analyzer with the PSArray2 a cost-effective alternative to the Bispectral Index Monitor during the perioperative period? Anesth Analg. 2004;99:1429-1435.
3. Drover DR, Lemmens HJ, Pierce ET, et al. Patient State Index: titration of delivery and recovery from propofol, alfentanil, and nitrous oxide anesthesia.Anesthesiology. 2002;97:82-89.
4. Cassingham SF, Hebert T, Lemaire R, Cook R, McPherson PK. The Physiometrix PSA 4000 decreases propofol usage and hastens discharge in gynecological day surgery procedures. Anesthesiology. 2002;96:A5.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that SEDLine facilitates more individualized titration of anesthesia that leads to patients receiving significantly less anesthetic and recovering faster, risks related to our assumptions of the repeatability of clinical results obtained, and risks related to our assumptions that SEDLINE will facilitate immediate detection of asymmetrical activity in all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Announces Adoption of Stock Repurchase Program
Irvine, California – August 9, 2011 – Masimo (NASDAQ: MASI) today announced that its Board of Directors has authorized the repurchase of up to 3 million shares of the company's common stock. The stock repurchase program may be carried out at the direction of the company through open market purchases, block trades, and in privately negotiated transactions. The repurchase program will become effective on August 12, 2011 and is expected to continue for a period of 24 months unless it is terminated earlier by the Board of Directors. Any repurchases will be subject to the availability of stock, general market conditions, the trading price of the stock, alternative uses for capital and the company's financial performance. The company expects to fund the stock repurchase program through its available cash.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said: "This stock repurchase program underscores our continued belief and optimism in Masimo's long-term growth prospects, as well as our commitment to return value to our stockholders. Our management team and Board of Directors have a strong conviction in our growth prospects, business model and cash flow generation capability, and we believe that the stock represents an attractive value relative to our future outlook."
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements, including statements that the company will generate future cash flow, the repurchase of company stock constitutes an opportunity to increase stockholder value, available cash should provide sufficient liquidity to support the stock repurchase program, and the repurchase of our stock represents an attractive investment. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce the anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws. The repurchase program authorization does not require the company to purchase a specific number of shares and the program may be modified, suspended or terminated at any time. The timing and number of shares repurchased, if any, pursuant to the stock repurchase authorization will be subject to a number of factors, including current market conditions, legal constraints and available cash or other sources of funding. This press release is not an offer to purchase any securities.
Investor Contact:
Sheree Aronson
Vice President,
Investor Relations, Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Director,
Public Relations, Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation


Masimo Reports Second Quarter 2011 Financial Results
Irvine, California, August 9, 2011 – Masimo (NASDAQ: MASI) today announced its financial results for the second quarter ended July 2, 2011.
Q2 2011 Highlights (compared to Q2 2010):
- Total revenue, including royalties, rose 9% to $109.6 million
- Product revenue rose 17% to $102.6 million
- Masimo SET®and Masimo rainbow®SET unit shipments rose 2% to 37,300
- Masimo rainbow revenue rose 26% to $9.1 million
- GAAP EPS was $0.28 versus $0.24 in the prior period, which included $0.01 in one-time expenses. Excluding the prior period one-time items, GAAP EPS rose 12% from an adjusted $0.25 in Q2 2010
Masimo's total revenue, including royalties, for the second quarter rose 9% to $109.6 million, compared to $100.1 million for the second quarter of 2010. Masimo's second quarter product revenue rose 17% to $102.6 million, compared to $88.0 million for the second quarter of 2010. Revenue from Masimo rainbow products rose 26% to $9.1 million in the second quarter, compared to $7.2 million for the second quarter of 2010.
Net income for the second quarter was $17.0 million, or $0.28 per diluted share, compared to reported net income of $14.3 million, or $0.24 per diluted share, in the second quarter of 2010, which included $0.01 per diluted share in one-time marketing-related expenses. Excluding these one-time expenses, diluted earnings per share rose 12% in the second quarter of 2011 from the year-ago period.
During the second quarter, the company shipped approximately 37,300 Masimo SET pulse oximetry and Masimo rainbow SET Pulse CO-Oximetry units, excluding handheld units, up 2% compared to approximately 36,700 in the same prior year period. Masimo estimates its worldwide installed base as of July 2, 2011 to be 922,000 units, up 17% from 789,000 units as of July 3, 2010.
As of July 2, 2011, cash and cash equivalents was $125.7 million, compared to $88.3 million as of January 1, 2011.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 78780959. After the live webcast, the call will be available on Masimo's website through September 9, 2011. In addition, a telephonic replay of the call will be available through August 16, 2011. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 78780959.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about: our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies; and global demand for our technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact:
Sheree Aronson
Vice President,
Investor Relations, Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Director,
Public Relations, Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.



Masimo Introduces Pronto-7™ Internationally and Lifts Voluntary Recall
 |
|
The palm-sized Pronto-7, with dimensions of just 13 cm x 7.2 cm x 2.5 cm (5.1" x 2.8" x 1") and weight of 296 grams (10.5 ounces), offers a breakthrough solution for measuring hemoglobin, SpO2, pulse rate, and perfusion index in less than one minute—without the needles, time-consuming laboratory analysis, risk of blood contamination, hazardous medical waste, and patient discomfort associated with traditional blood tests. |
Irvine, California – August 8, 2011 – Masimo (NASDAQ: MASI) announced today that it has lifted the voluntary product recall the company imposed on the noninvasive finger sensor of its Pronto-7™ handheld device to improve the product's performance in low ambient temperatures. The Pronto-7 and new finger sensors are pending FDA 510(k) clearance in the U.S.
The palm-sized Pronto-7, with dimensions of just 13 cm x 7.2 cm x 2.5 cm (5.1" x 2.8" x 1") and weight of 296 grams (10.5 ounces), offers a breakthrough solution for measuring hemoglobin, SpO2, pulse rate, and perfusion index in less than one minute—without the needles, time-consuming laboratory analysis, risk of blood contamination, hazardous medical waste, and patient discomfort associated with traditional blood tests.
New product features and enhancements include the addition of Max Sensitivity Mode that allows measurement over a broader range of patients. The addition of three noninvasive sensor sizes permits the instrument to be used on a broader range of finger diameters. Each of the new sensors is color-coded to make size identification easy (Small-Yellow, Medium-Red, and Large-White).


Masimo Founder and CEO, Joe Kiani, stated: "We take the integrity of our design and our promises to our customers seriously. We worked through the recall until we had redesigned the sensor for Pronto-7 so that it would perform as intended in the broadest range of likely ambient temperatures. We are delighted to re-introduce the Pronto-7 and with it the potential impact of noninvasive hemoglobin spot-check measurements that offer healthcare providers around the world the opportunity to transform the way and amount of time it takes to deliver the right care, at the right time to patients. We believe that Pronto-7's latest features and enhancements will help clinicians to better assess more patients and make better clinical decisions based on the availability of these quick and noninvasive hemoglobin measurements."
As part of Masimo's verification process, over 11,443 Pronto-7 measurements were performed on 1,445 subjects at 14 sites and compared to hemoglobin measurements from a venous blood sample analyzed on a hematology analyzer (Coulter Counter). This testing resulted in a product specification of 1.0 g/dL for normal sensitivity mode and 1.1 g/dL for max sensitivity mode. While not a regulatory requirement, prior to lifting the voluntary recall Masimo elected to perform additional testing on Pronto-7 devices and sensors produced at a Masimo manufacturing facility. This additional testing included 474 subjects in three outpatient clinic-type environments. The hemoglobin measurements from the Pronto-7 and a point of care device using capillary blood (Hemocue) were compared to hemoglobin measurements from a venous blood sample analyzed on a Coulter Counter. The Hemocue showed a bias of -0.1 g/dL and a standard deviation of 1.6 g/dL, while the Pronto-7 showed a bias of was -0.1 g/dL and a standard deviation of 1.1 g/dL. Based on the clinical results obtained and positive feedback from clinicians, Masimo has initiated international availability of Pronto-7 in Europe, Middle East, Africa, South America, and Asia (except for countries requiring clearance, such as Japan).
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic MonitoringTM, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that repositioning the thermistor solves prior temperature measurement problems, risks related to our assumptions of the repeatability of clinical results obtained using the enhanced Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Report Second Quarter 2011 Financial Results after Market Close on August 9, 2011
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., July 19, 2011 -- Masimo (NASDAQ: MASI) announced today that it will release second quarter financial results for the period ended July 2, 2011, after the market closes on August 9, 2011. The release and conference call date is approximately one week later than Masimo's traditional release timing due to executive calendar conflicts during the month of July.
The conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 78780959. After the live webcast, the call will be available on Masimo's website through September 9, 2011. In addition, a telephonic replay of the call will be available through August 23, 2011. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 78780959.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo and Dartmouth-Hitchcock Medical Center, First Cross-Industry Collaborative Honored with the Institute for Technology and Healthcare Clinical Application Award
The Association for the Advancement of Medical Instrumentation (AAMI) Foundation Award Honors the Clinical Application and Efficacy Excellence Demonstrated Through Implementation of Masimo Patient SafetyNet™
Irvine, California – June 27, 2011 – Masimo Corporation (NASDAQ: MASI) announced today that two of the industry's top patient safety collaborators—James Welch, VP of Patient Safety Initiatives at Masimo, and George Blike, Medical Director of Patient Safety Training at Dartmouth-Hitchcock Medical Center—are the recipients of the AAMI Foundation'sInstitute for Technology and Healthcare Clinical Application Award. Awarded each year to an individual or group who has applied innovative clinical engineering practices or principles to solve patient care problems through demonstrated clinical application and efficacy excellence, this is the first time in the association's history that the award was shared between two cross-industry collaborators—a medical technology innovator/developer (Masimo) and a patient care provider/hospital facility (Dartmouth-Hitchcock Medical Center).
Honored for their cross-industry collaboration in the implementation of Masimo Patient SafetyNet™ at Dartmouth-Hitchcock, Welch (Masimo) and Blike (Darmouth-Hitchcock) combined their high-tech and human-touch expertise to install the remote monitoring and wireless clinician notification system based on Masimo SET®Measure-Through Motion and Low Perfusion pulse oximetry monitoring that led to a "significant drop" in key clinical outcome measures—including 65% fewer rescue events, 48% fewer ICU transfers, and reduced annualized ICU time by 135 days.1
"James Welch (with Masimo) and George Blike (with Dartmouth-Hitchcock) exemplify the term effective collaboration," stated Mary Logan, President of the Association for the Advancement of Medical Instrumentation. "What is particularly powerful about this duo is that they have modeled for all of us what can be accomplished when you have an effective collaboration between clinical engineering and front-line clinicians, between industry and patient safety experts, and between technology developers and a clinician who knows how to assess in a clinical setting the impact of a technology solution to a vexing safety problem. We can all be grateful for the power of what they have accomplished here."
Designed to improve patient safety through continuous pulse oximetry monitoring, Patient SafetyNet keeps general care floor patients safer by continuously, noninvasively, and remotely monitoring multiple physiological parameters, including arterial oxygen saturation and pulse rate, and automatically alerting clinicians to changes that signal patient distress or deterioration via pager or phone. The system's alarm escalation process is an important feature that notifies additional clinicians if a life-threatening alarm persists—ensuring that alarms are escalated to other clinicians in the event the assigned primary clinician is busy or unresponsive. With the system in place, Dartmouth-Hitchcock clinicians receive a pager notification when a patient's condition is worsening—allowing them to intervene before the condition becomes critical and requires more acute levels of care. This is particularly important for post-surgical patients who are at increased risk of serious injury or death resulting from the respiratory depression effects of patient-controlled analgesia (PCA) and opioids used for sedation and pain management.
The AAMI Foundation Awards Committee performs rigorous reviews of candidates who have demonstrated outstanding service or accomplishment with a significant impact on a specific medical device or on medical instrumentation in general, a service that results in the development of an important new medical device, a distinctive contribution toward the improvement of the use or safety of a medical device, or critical contributions to the enhancement of patient care through medical instrumentation. Their recognition of the significant impact made by both Welch and Blike in the cross-industry collaboration to improve patient safety on the general care floor with Patient SafetyNet is proof-positive that the combination of high-tech medical technology expertise and high-touch clinical care expertise are a winning pair for patients.
The Foundation'sprimary mission is to serve public welfare and improve patient safety by fostering support and recognition for the advancement of medical technology. It does this through recognition awards for excellence, scholarships, global outreach, and the Medical Device Safety Council. The Foundation's Excellence Awards recognize individuals and groups for their superior work in medical instrumentation development, research, and applications that improve public health care safety, as well as humanitarian efforts that apply health technology to improve global human conditions.
1 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Available online at: http://journals.lww.com/anesthesiology/Abstract/publishahead/Impact_of_Pulse_Oximetry_Surveillance_on_Rescue.99692.aspx
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using Masimo rainbow SET measurements, risks related to our belief that rainbow measurements can help clinicians more rapidly assess patients to detect adverse conditions earlier, including the early signs of hemodynamic instability, potentially life-threatening conditions, and internal bleeding, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


New Clinical Research Presented at the European Society of Anaesthesia Demonstrates the Accuracy and Utility of Masimo SpHb and PVI
Irvine, California – June 16, 2011 – Masimo Corporation (NASDAQ: MASI) announced today that multiple new clinical research studies presented this week at the European Society of Anaesthesiology (ESA) Annual Congress in Amsterdam add to the growing body of evidence that Masimo noninvasive and continuous total hemoglobin (SpHb®) and Pleth Variability Index (PVI®) are accurate and useful noninvasive and continuous measurements in a variety of patients.
Two studies, from researchers in Japan and Sweden, evaluated the accuracy and reliability of SpHb in both surgical and ED patients and found clinically acceptable correlations between noninvasive SpHb measurements and invasive lab values. Researchers at the Hamamatsu University School of Medicine in Japan analyzed 89 hemoglobin samples from 16 surgical patients and found a correlation coefficient between SpHb (obtained using the Masimo Radical-7 and R-125 rev C sensor) and invasive lab values of 0.8 with a bias of 0.65 g/dL and precision of 1.1 g/dL. Citing this as the first introduction of SpHb to Japanese patients, researchers concluded that "SpHb is reliable on Japanese patients." 1 Similarly, researchers at the Karolinska Institute in Stockholm, Sweden, compared SpHb measurements (obtained using the Masimo Radical-7 software version 7.6.0.1 and adult ReSposable sensor rev E) to invasive venous blood sampling (tHb) in 30 patients admitted to the emergency room and found a very low bias of -0.2 and standard deviation of 1g/dL, with limits of agreement of -2.2 to 1.8 g/dL. Citing "fairly good agreement between tHb and SpHb values across the entire measurement range," researchers concluded that "SpHb monitoring provides hemoglobin values with good agreement to laboratory analysis of blood."2
Two other studies, conducted in France and Japan, analyzed finger, ear, and forehead sites for PVI measurement and found that sites on the head enable the best measurement. Previous studies have shown that PVI, as measured at the finger site, can help clinicians assess fluid responsiveness and improve fluid management to decrease patient risk. Results showed that Masimo ear and forehead sensors provide the best accuracy for PVI measurement. At the Louis Pradel Hospital in France, researchers evaluated PVI measurements obtained from the ear, forehead, and finger (using the Masimo Radical-7 and LNOP Adt sensor) in 16 patients during ongoing surgery and found that PVI correlations to pulse pressure variations (PPV) were r=0.72 at the ear, r=0.60 at the forehead, and r=0.43 at the finger (all p<0.001). PVI forehead >14% and PVI ear >15% predicted a PPV > 10% with 73% sensitivity and 72% specificity, while PVI finger > 13% predicted a PPV > 10% with a sensitivity of 78% and a specificity of 44%—leading researchers to conclude that "ear and forehead provide best accuracy for PVI."3 In Japan, researchers at Kagoshima University tested the effects of surgical stimuli on PVI measurements at the finger and forehead (obtained using the Masimo Radical-7 software version 7.3.11 and LNOP Adt sensor) in nine mechanically ventilated surgical patients and found that forehead PVI & perfusion index (PI) remained stable before and after skin incision while PVI captured at the finger site were more sensitive to vasomotor tone. Researchers concluded that "PVI obtained from the forehead may be a more reliable predictor of fluid responsiveness under conditions of varying vasomotor tone."4
SpHb and PVI are available as part of Masimo rainbow SET®platform—the first-and-only technology to noninvasively and continuously measure total hemoglobin (SpHb®) , oxygen content (SpOC™), carboxyhemoglobin (SpCO®) , methemoglobin (SpMet®) , Pleth Variability Index (PVI®) , perfusion index (PI), and acoustic respiration rate (RRa™) , in addition to the 'gold standard' Measure-Through Motion and Low Perfusion performance of Masimo SET®oxyhemoglobin (SpO2), and pulse rate (PR).
To see a summary of all known clinical studies and abstracts on Masimo SpHb, please visit: https://professional.masimo.com/evidence/featured-studies/feature/.
The ESA 2011 schedule of clinical abstracts presented can be accessed online at http://www.euroanaesthesia.org/sitecore/Content/Congresses/Euroanaesthesia%202011/~/media/Congress/2011%20Amsterdam/Scientific%20Programme/2011_Abstract%20Sessions_Day%20by%20Day_11-05-2011.ashx.
1 Ishida C, Shiraishi Y., Mimuro S., Yu S., Katoh T., Sato S. "Accuracy of a Noninvasive Measurement of Hemoglobin Via Pulse CO-Oximetry in Japanese Population." Hamamatsu University School of Medicine, Department of Anesthesiology and Intensive Care, Hamamatsu, Japan.
2 Svensen C.H., Rodhe P., Lundstrom N., Berglund E., Sjostrand F. "Use of a Noninvasive Hemoglobin Monitor for Volume Kinetic Analysis in an Emergency Room Setting." Karolinska Institute,/Department of Clinical Science and Education, Department of Anesthesiology and Intensive Care, Stockholm, Sweden.
3 Pavlakovitch I., Desebbe O., Cannesson M., Bastien O., Lehot J.J. "What is the Best Site for Measuring the Pleth Variability Index (PVI) During a Surgery Procedure?" Hospices Civils de Lyon Hopital L Pradel, Department of Anesthesiology and Intensive Care, Bron-Lyon, France.
4 Matsunaga A., Kakihan Y., Kuniyoshi T., Kumemura M. Uchida Y., Kanmura Y. "Difference Between Pleth Variability Index Obtained from the Finger and Forehead." Kagoshima University, Department of Anesthesiology, Kagoshima, Japan.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using Masimo SpHb and PVI noninvasive measurements, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Announces Rainbow Agreement with Philips
Irvine, California June 15, 2011 – Masimo (NASDAQ: MASI) today announced that it has reached a long-term non-exclusive agreement under which Philips will integrate Masimo rainbow SET®technology into Philips' products. Masimo expects to begin diligent co-development projects with Philips, which should allow rainbow SET technology to be available in Philips' products worldwide.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our Rainbow agreement with Philips. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce the anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Pronto-7 Wins GOLD in the 2011 Medical Design Excellence Award Competition—the Premier Awards Program for the Medical Technology Community
 The palm-sized Masimo Pronto-7™ offers noninvasive hemoglobin, SpO2, pulse rate, and PI spot-check results. The palm-sized Masimo Pronto-7™ offers noninvasive hemoglobin, SpO2, pulse rate, and PI spot-check results. |  |
|---|
Irvine, California – June 9, 2011 – Masimo Corporation (NASDAQ: MASI) announced today that its Pronto-7™ handheld noninvasive hemoglobin, arterial oxygen saturation (SpO2), pulse rate, and perfusion index spot-check testing device has received the GOLD 2011 Medical Design Excellence Award—the highest award given in the general hospital devices and therapeutic products category. The 2011 Medical Design Excellence Award winners were honored yesterday at a presentation ceremony in New York City's Jacob K. Javits Convention Center, in conjunction with the Medical Design & Manufacturing (MD&M) East Conference and Exposition (www.MDMEast.com).
The Medical Design Excellence Awards competition (www.MDEAwards.com) is organized and presented by UBM Canon (Los Angeles) and is the only awards program that exclusively recognizes contributions and advances in the design of medical products. Entries are evaluated on the basis of their design and engineering features, including innovative use of materials, user related functions that improve healthcare delivery and change traditional medical attitudes or practices, features that provide enhanced benefits to the patient, and the ability of the product development team to overcome design and engineering challenges so that the product meets its clinical objectives. A comprehensive review of the entries was performed by an impartial, multidisciplinary panel of third-party jurors with expertise in biomedical engineering, human factors, industrial design, medicine, and diagnostics.
The Pronto-7 is truly an incredible breakthrough for clinical users. The palm-sized device was designed for quick-and-easy noninvasive hemoglobin (SpHb®) spot-check testing, along with SpO2, pulse rate, and perfusion index. Extremely lightweight at approximately 296g, the Pronto-7 delivers total hemoglobin measurement results—a physiological parameter that previously required an invasive blood draw. Offering needle-less and pain-free hemoglobin blood testing, the Pronto-7 is a cost-effective solution for immediate, on-the-spot hemoglobin testing that is raising the expectations of patients and clinicians alike to more advanced healthcare at the earliest moment of care.
"We are honored that the judges have selected Pronto-7 as a GOLD MDEA winning product," said Joe Kiani, Founder and CEO of Masimo. "Pronto-7 was designed to help overcome many of the challenges and risks associated with invasive hemoglobin sampling for both clinicians and patients—enabling fast, accurate, painless, needle- and risk-free total hemoglobin measurements."
The new Pronto-7 with small, medium, and large size finger sensors will soon be launched internationally and is pending FDA 510(k) Clearance in the U.S.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that Masimo Pronto-7 delivers noninvasive hemoglobin measurements in under one minute for all patients, and risks related to our belief that Pronto-7 provides needle-less and pain-free hemoglobin blood testing for clinicians in virtually any environment, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at William Blair & Company 31st Annual Growth Stock Conference
IRVINE, Calif., June 3, 2011 - Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the William Blair & Company 31st Annual Growth Stock Conference at the Four Seasons Hotel in Chicago on Wednesday, June 15, at 8:00 a.m. Central Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Initiates Limited Market Release of E1, Single-Patient-Use Ear Sensor for Pulse Oximetry Monitoring
Providing an Alternative Monitoring Site in the Ear Conchae for Faster Detection of Oxygen Saturation Changes
Irvine, California – June 2, 2011 – Masimo (NASDAQ: MASI) announced today FDA 510(k) Clearance, CE Mark, and limited market release of the industry's first single-patient-use ear sensor. Compared to digit sensors, the Masimo E1™ enables faster detection of oxygen saturation changes during low perfusion due to a variety of clinical factors, including sedative or medication-induced vasoconstriction.1,2,3 Compared to reusable ear sensors, it also avoids cross-contamination risks for patients and reduces the complexity of sensor management for clinicians, including cleaning, storage, and transport. In the limited market release, select clinicians around the world will have the opportunity to use and evaluate the performance and benefits of the E1 sensor.
Due to the signal processing limitations of conventional pulse oximetry during challenging conditions, clinicians often seek alternative monitoring sites on the head. In spite of the established need, available single-patient-use sensors for the head have suffered from unreliability. While Masimo SET Measure-through Motion and Low Perfusion pulse oximetry overcomes the limitations of conventional pulse oximetry and offers reliable true alarm detection and false alarm prevention during challenging conditions, there are still some advantages to monitoring on the head such as faster response to oxygenation changes during low perfusion and the use of an alternative site when the digit is unavailable.
 The Masimo E1™ Ear Sensor is placed conveniently and securely in the cavum conchae (inner aspect of the ear). |
|---|
As a single-patient-use ear sensor that is placed securely in the cavum conchae (the deep hollow near the ear canal opening), the E1 allows clinicians to combine Masimo SET performance with a reliable alternative monitoring site while minimizing cross-contamination. This site frees up the patient's hands and is easy-to-access for clinicians. Made of durable soft silicone, it has no moving parts that can entangle in the hair or irritate the skin. In addition to its advantages for faster detection of saturation changes during low perfusion, it also offers an alternative site when other monitoring sites are unavailable due to emergency transport or use by another type of head sensor such as those used for Masimo's SEDLine®brain function monitoring. In addition, by using the cavum conchae as the monitoring site, the E1 avoids the challenges associated with forehead sensors, including known limitations in the presence of venous pulsations and when patients are in a supine or trendelenburg position. When compared to the traditional ear lobe site, the cavum conchae site also offers better perfusion and superior signal quality—enabling improved monitoring performance in a sensor that stays in place more securely.
According to Dr. Daniel Davis, a contributor on the design of the E1 ear sensor, Professor of Clinical Emergency Medicine, and Director of the UCSD Center for Resuscitation Science in San Diego, California, "The Masimo E1 ear sensor is an ideal monitoring site solution for multiple clinical applications. With it, signal detection is optimized in low perfusion states (sepsis, hemorrhage, hypothermia) and in the presence of peripheral vascular disease. In my experience, the E1 ear sensor detected hypoxemia up to 2-3 minutes sooner, which is critically important during airway management, resuscitation, and inpatient apnea/hypopnea episodes. Additionally, the ear site is more accessible and offers distinct advantages when traditional peripheral monitoring sites are unavailable due to trauma or injury, are swollen and do not easily fit into a digital sensor, or are inaccessible due to drapes, bandages, and dressings. The E1 also eliminates the need for excess cords making it ideal for ambulatory and general care floor patients because it frees up their hands for better mobility, physical therapy, or exercise."
To locate a Masimo representative or distributor in your area and evaluate the E1 sensor, call 1-800-257-3810 or visit www.masimo.com.
1Tokuda K, Hayamizu K, Ogawa K, Hirai T, Irita K. "A Comparison of Finger, Ear and Forehead SpO2 on Detecting Oxygen Desaturation in Healthy Volunteers." Anesthesiology 2007; 107: A1544.
2 Redford D, Lichtenthal P, Barker SJ. "Evaluation of Masimo SET Ear and Forehead Pulse Oximetry and Nellcor MAX-FAST Forehead Pulse Oximetry." Anesthesiology 2004; 101: A593 and A579.
3 Whitman RA, Garrison ME, Oestreich TJ, Musumbi MS. "Evaluation of a New Reflectance Forehead Sensor in Detecting Oxygen Desaturation in Patients Undergoing Polysomnography." Anesthesiology 2003; 99: A553.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the Masimo E1 disposable ear sensor provides faster response times and rapid detection of oxygen saturation, risks related to our assumptions that the E1 sensor offers improved performance for patients in extremely low perfusion states, and risks related to our belief that the design of the E1 sensor does not put excessive pressure on the site and eliminates cross contamination risks, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at Goldman Sachs 32nd Annual Global Healthcare Conference
IRVINE, Calif., May 26, 2011 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the Goldman Sachs 32nd Annual Global Healthcare Conference at Terranea in Rancho Palos Verdes, California, on Thursday, June 9, 2011, at 8:40 a.m. Pacific Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Launches MS-2040, First Sub-45 mW Signal Extraction Pulse Oximetry Platform
New MS-2040 Circuit Board and External "Board-in-Cable" Promises to Usher in Breakthrough Pulse Oximetry Applications
Irvine, California – May 19, 2011 – Masimo (NASDAQ: MASI) announced today the launch of its new sub-45 milliwatt technology platform—MS-2040. This platform utilizes nearly a third of the power and half of the physical board size of previously available Signal Extraction Pulse Oximetry solutions while providing the same proven unprecedented Measure-Through Motion and Low Perfusion oxygen saturation (SpO2), pulse rate, and perfusion index (PI) measurement capabilities of Masimo SET®pulse oximetry, in addition to Pleth Variability Index (PVI).
  |
|---|
| CAPTION: The compact size and reduced power consumption of the Masimo MS-2040 double-stacked circuit board enables a broad range of reliable small, lightweight, handheld and wearable portable medical device possibilities. |
Using less than 45 milliwatts of power, Masimo MS-2040 boards are available in both a double-stack version (pictured) with dimensions of 1.8" x 1.15" x 0.46" and single-stack version with dimensions of 2.0" x 1.38" x 0.36". The small size and reduced power consumption of Masimo MS-2040 boards enhance integration into next-generation portable medical devices that need to be smaller, lighter, and more mobile—consuming less than a third of the power without sacrificing accuracy during motion and low perfusion.
According to Masimo President of Worldwide OEM Business and Corporate Development, Rick Fishel, "Over the last 15 years, Masimo has worked diligently to continuously enhance pulse oximetry features and performance, including the development of cutting-edge circuitry innovations that have reduced the size of our boards by 90% and power consumption by 98%. Reducing the power usage profile and physical size of our circuit boards enables Masimo and our OEM partners to design smaller, lighter, and more portable monitoring devices with the high performance pulse oximetry capabilities clinicians need for successful utilization in these new sites and applications."
The MS-2040 circuit board is also available as an external "board-in-cable" solution. Embedded within a completely self-contained patient cable, the "board-in-cable" solution provides external Masimo SET pulse oximetry capabilities for existing devices that do not presently offer an integrated pulse oximetry solution. This affords medical equipment manufacturers a simpler way to add Masimo SET pulse oximetry as an external (oximeter outside) option and enables a wide range of reliable handheld and patient-wearable application possibilities.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the high performance and low power consumption capabilities of the Masimo MS-2040 technology platform, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, MS-2040, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


New Published Study Demonstrates the Accuracy of Masimo Noninvasive and Continuous Hemoglobin Monitoring in Patients in Surgery and Intensive Care
Study Authors Indicate Masimo SpHb Allows for More Rapid and Appropriate Medical Interventions
Irvine, California – May 16, 2011 – Masimo (NASDAQ: MASI) announced today that findings from a new study, published in the American Journal of Surgery,demonstrate that noninvasive and continuous hemoglobin (SpHb®) monitoring correlates strongly with invasive laboratory hemoglobin testing and "can be beneficial in the operating room and critical care setting and may potentially decrease the invasiveness of both elective surgery and critical illness."1 This is the first study to examine the accuracy and reliability of Masimo SpHb for measuring hemoglobin and hemorrhage in both the operating room and intensive care unit (ICU).
Total hemoglobin measurement is one of the most frequently ordered laboratory tests. However, hemoglobin levels must be obtained through blood draws that are invasive, time-consuming, and can only provide data at one point in time. Since the introduction of pulse oximetry, monitoring of blood oxygenation in the operating room has become a standard of care. Today with the Masimo rainbow SET®platform, clinicians now have access to noninvasive and continuous pulse oximetry along with SpHb and other blood constituent parameters— all in one device and from one sensor.
In the study, conducted at Madigan Army Medical Center in Tacoma, Washington, researchers compared noninvasive SpHb and invasive laboratory measurements in a series of patients undergoing major surgical procedures in the operating room or with severe illnesses in the ICU. Using the Radical-7®Pulse CO-Oximeter, measurements were obtained on 25 elective surgical patients in the operating room and 45 critically ill patients in the ICU and compared to hemoglobin measurements obtained invasively every hour from the radial artery catheter. A total of 27 point-of-care invasive hemoglobin measurements (iStat®from Abbott) were also collected. Significant bleeding was identified in 44% of the surgical patients.
Results showed an overall correlation between SpHb and lab values of 0.78 (P < .001) with a mean difference of 0.15 g/dL (95% confidence interval, -0.03 to 0.32 g/dL). In the OR, the mean difference was 0.29 g/dL (95% confidence interval, 0.09 to 0.50g/dL) while in the ICU, the mean difference was 0.05 g/dL (95% confidence interval, 0.22 to 0.31 g/dL). In the five patients requiring intra-operative blood transfusion, there was a very strong correlation of 0.91 between SpHb and lab measurements. When the point-of-care measurements from iStat were compared to lab measurements, the results were similar to SpHb comparisons to lab measurements, with a correlation of 0.85 and a mean difference of 0.09 g/dL (95% confidence interval 0.17 to 0.34 g/dL).
As a result of these findings, researchers deemed that "noninvasive hemoglobin monitoring is especially important" for high-risk critically ill patients or patients undergoing high-risk operations, critically ill bleeding/transfused patients or patients undergoing operations with an expected high blood loss, and for patients not expected to have significant bleeding intra-operatively but in whom obtaining laboratory values would be difficult or impossible if not pre-planned.
Commenting that SpHb monitoring "will benefit the patient, intensivist, and anesthesiologist by allowing continuous physiological monitoring without having to occlude the arterial line and may even preclude the need for a central line when the purpose is only for frequent laboratory hemoglobin analysis," researchers concluded that "based on the strong correlation between SpHb and laboratory hemoglobin, noninvasive hemoglobin monitoring can be beneficial in the operating room and critical care setting and may potentially decrease the invasiveness of both elective surgery and critical illness while providing continuous hemoglobin monitoring in addition to standard pulse oximetry data."
SpHb is available as part of Masimo rainbow SET®platform—the first-and-only technology to noninvasively and continuously measure total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), perfusion index (PI), and acoustic respiration rate (RRa™), in addition to the 'gold standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), and pulse rate (PR).
1 Causey MW, Miller S, Foster A, Beekley A, Zenger D, Martin M. "Validation of noninvasive hemoglobin measurements using the Masimo Radical-7 SpHb Station." Am J Surgery 2011: 201: 590-596. For further reading, visit: http://www.americanjournalofsurgery.com/article/S0002-9610(11)00119-X/abstract.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SpHb is capable of providing continuous physiological monitoring of hemoglobin levels in all surgical or critically ill patients, and risks related to our assumptions that Masimo SpHb monitoring has the potential to decrease the invasiveness of elective surgery and critical illness, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Reports First Quarter 2011 Financial Results
Product revenue exceeds $100 million for first time in company's history
Q1 2011 Highlights (compared to Q1 2010):
- Total revenue, including royalties, rose 14% to $113.0 million
- Product revenue rose 18% to $101.6 million
- Masimo SET®and Masimo rainbow®SET unit shipments rose 16% to 43,100
- Masimo rainbow revenue rose 39% to $7.4 million
- GAAP EPS was $0.30 compared to $0.44 in Q1 2010, which included $0.20 in one-time items related to the antitrust lawsuit victory in Q1 2010. Excluding these one-time items, GAAP EPS of $0.30 was up 25% from an adjusted EPS of $0.24 in Q1 2010
Irvine, California, May 3, 2011 – Masimo (NASDAQ: MASI) today announced its financial results for the first quarter ended April 2, 2011.
Masimo's total revenue, including royalties, for the first quarter rose 14% to $113.0 million, compared to $98.8 million for the first quarter of 2010. Masimo's first quarter product revenue rose 18% to $101.6 million, compared to $85.9 million for the first quarter of 2010. Revenue from Masimo rainbow products rose 39% to $7.4 million in the first quarter, compared to $5.3 million for the first quarter of 2010.
Net income for the first quarter was $18.0 million, or $0.30 per diluted share, compared to reported net income of $26.7 million, or $0.44 per diluted share, in the first quarter of 2010 and compared to adjusted net income of $14.3 million, or $0.24 per diluted share, in the first quarter of 2010. Adjusted earnings per share in the first quarter of 2010 excludes $0.20 per diluted share from the net of a $30.1 million one-time gain related to an antitrust lawsuit victory and offset by $10.9 million in one-time grants and marketing initiatives. One-time grants in the first quarter of 2010 included a $10.3 million donation to establish the Masimo Foundation for Ethics, Innovation and Competition in Healthcare.
During the first quarter, the company shipped approximately 43,100 Masimo SET pulse oximetry and Masimo rainbow SET Pulse CO-Oximetry units, excluding handheld units, up 16% compared to approximately 37,100 in the same prior year period. Masimo estimates its worldwide installed base as of April 2, 2011 to be 890,000 units, up 18% from 757,000 units as of April 3, 2010.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "We continue to experience increasing global demand for our breakthrough technologies as evidenced by our new highs in product revenue and driver shipments in the quarter. We continue to pursue our mission to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications."
As of April 2, 2011, cash, cash equivalents and short-term investments totaled $105.6 million, compared to $88.3 million as of January 1, 2011
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 58223656. After the live webcast, the call will be available on Masimo's website through June 3, 2011. In addition, a telephonic replay of the call will be available through May 17, 2011. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 58223656.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about: our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies; and global demand for our technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations,
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.

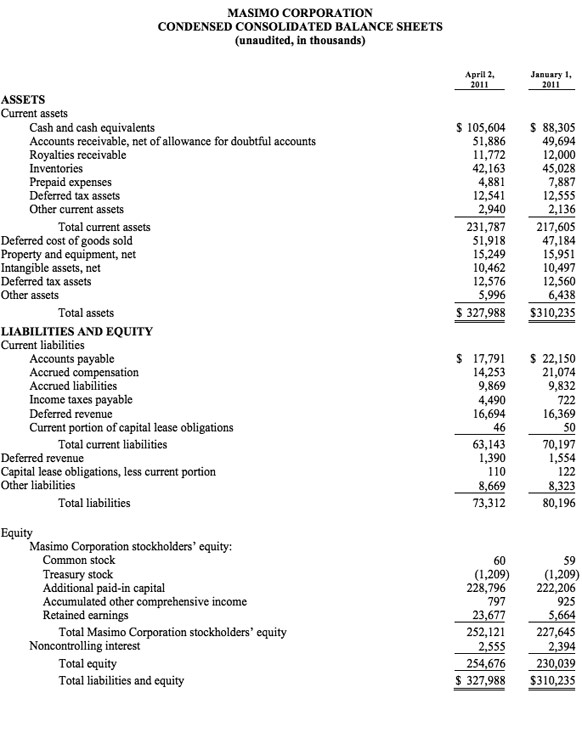
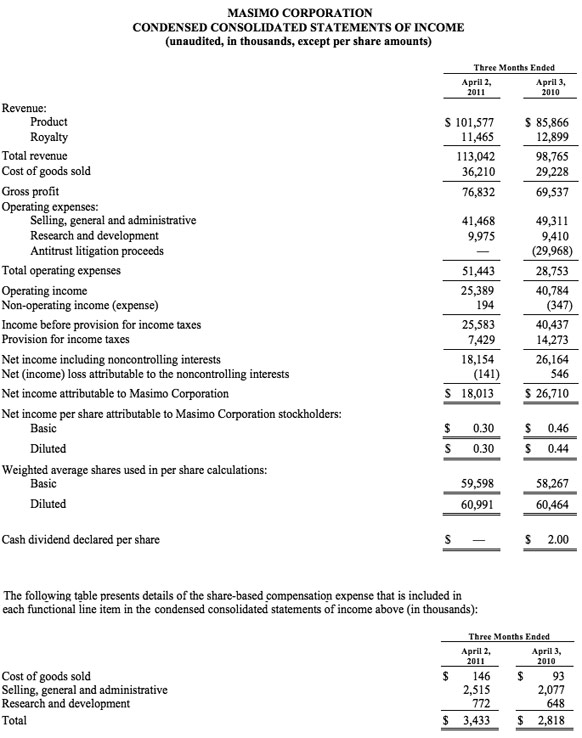
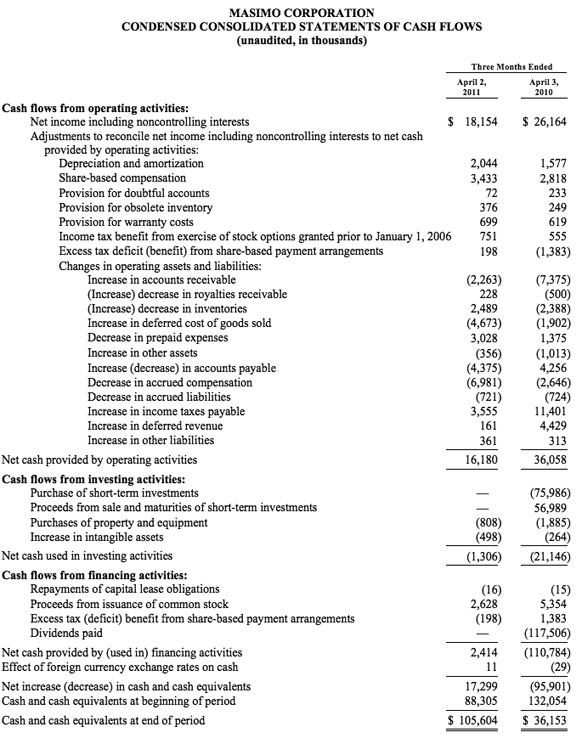


Masimo Launches New Reusable SpCO® Sensor with Improved Accuracy and Reliability in Low Oxygen Saturation and Elevated Methemoglobin States
Irvine, California – April 28, 2011 – Masimo Corporation (NASDAQ: MASI) announced today the availability of new, enhanced versions of its reusable, noninvasive carboxyhemoglobin (SpCO) sensors—with improved accuracy and reliability performance for patients with low oxygen saturation (SpO2) between 90% and 95% and elevated methemoglobin (SpMet®) between 0% and 2%.
Low SpO2 and elevated SpMet states are known physiological factors that compromise SpCO accuracy and contribute to erroneous measurements. Although this limitation still applies, the new sensors will provide enhanced SpCO performance when a patient's functional SpO2 is > 90%, as well as when their SpMet is < 2%.
Carbon monoxide (CO) poisoning is a major cause of morbidity and mortality. At least 20,000 known exposures1 and 439 deaths2 a year are attributed to non-fire-related, unintentional CO poisoning cases in the U.S. However, large registry trials show the prevalence of CO poisoning is far greater with approximately 50,000 ED visits per year.3 Because the symptoms of CO poisoning are nonspecific—ranging from mild headache, nausea, confusion, and dizziness to end-organ injury, such as myocardial infarction, stroke, and death—diagnosis is difficult and has historically relied on clinical suspicion and confirmation by invasive blood gas analysis. Unfortunately, it has been estimated that up to half of U.S. hospitals do not have invasive COHb testing ability—increasing the potential that many victims of CO poisoning could be overlooked and misdiagnosed.4 Masimo SpCO allows immediate and noninvasive assessment of toxic CO levels—enabling early detection of CO poisoning that facilitates prompt, potentially life-saving treatment.
SpCO is part of the Masimo rainbow®SET platform—the first-and-only technology to noninvasively and continuously measure total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO), methemoglobin (SpMet), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the 'gold-standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR). Available in handheld (Masimo Rad-57™) and bedside devices (Masimo Radical-7™, Masimo Rad-87™), SpCO provides clinicians and emergency first responders with a way to quickly, noninvasively evaluate and address the significant health and safety risks associated with CO poisoning—helping to save and sustain lives, minimize suffering, and enhance safety. The new, enhanced versions of the Masimo rainbow Reusable Direct Connect SpCO and DCI®SpCO sensors (previous part numbers 2201, 2407, 2202, 2069, 2640, 2070, 2696, 2697) will now be identified as ID code H. Customers who would like to purchase the enhanced ID code H sensors should call (800) 257-3810 in the U.S., +41 32 720 1111 internationally, or their local sales representative.
1 Centers for Disease Control and Prevention. "Nonfatal, unintentional, non–fire-related carbon monoxide exposures—United States, 2004-2006." MMWR Morb Mortal Wkly Rep. 2008;57:896-899.
2 Centers for Disease Control and Prevention. "Carbon monoxide–related deaths—United States, 1999-2004." MMWR Morb Mortal Wkly Rep. 2007;56:1309-1312.
3 Keles A, Demircan A, Kurtoglu G. "Carbon monoxide poisoning: how many patients do we miss?" Eur J Emerg Med. 2008;15:154-157.
4 Hampson NB, Scott KL, Zmaeff JL. "Carboxyhemoglobin measurement by hospitals: implications for the diagnosis of carbon monoxide poisoning." J Emerg Med. 2006;31:13-16.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care… by Taking Non-invasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SpCO will provide an accurate and effective method of screening for CO poisoning in large numbers of at-risk populations, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at the 2011 Bank of America Merrill Lynch Health Care Conference
IRVINE, Calif., April 27, 2011 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the Bank of America Merrill Lynch Health Care Conference at the Encore at Wynn in Las Vegas on Wednesday, May 11, 2011, at 10 a.m. Pacific Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations,
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered


New Published Study Finds Masimo Noninvasive SpCO Effective for Screening Emergency Department Patients for Carbon Monoxide Poisoning
Irvine, California – April 21, 2011 – Masimo (NASDAQ: MASI) announced today that a new study published in this month's issue of the peer-reviewed journal, Annals of Emergency Medicine, demonstrates that noninvasive Masimo carboxyhemoglobin (SpCO®) measurements provide an "effective means for screening at-risk populations for CO poisoning" with "acceptable bias and precision" compared to invasive blood gas analysis. The prospective diagnostic accuracy study is more than ten times larger than any other published SpCO accuracy study to date and provides a strong rationale for clinical use of SpCO in the evaluation of emergency department (ED) patients.1
Carbon monoxide (CO) poisoning is a major cause of morbidity and mortality. At least 20,000 known exposures2 and 439 deaths3 a year are attributed to non-fire-related, unintentional CO poisoning cases in the U.S. However, large registry trials show the prevalence of CO poisoning is far greater with approximately 50,000 ED visits per year.4 Because the symptoms of CO poisoning are nonspecific—ranging from mild headache, nausea, confusion, and dizziness to end-organ injury, such as myocardial infarction, stroke, and death—diagnosis is difficult and has historically relied on clinical suspicion and confirmation by measurement of carboxyhemoglobin (COHb) via invasive blood gas analysis. Unfortunately, it has been estimated that up to half of U.S. hospitals do not have invasive COHb testing ability—increasing the potential that many victims of CO poisoning could be overlooked and misdiagnosed.5
The study, conducted over a year-long period in the Department of Emergency Medicine at one of the largest hospitals in Europe, the Vienna General Hospital (AKH Vienna), compared the accuracy of SpCO measured noninvasively using the Masimo Radical-7 with COHb measurements obtained via invasive blood gas analysis in 1,578 ED patients. Results showed a bias of 2.99% COHb (1.50% for smokers, 4.33% for nonsmokers) and a precision of 3.27% COHb (2.90% for smokers, 2.98% for nonsmokers) between SpCO and lab values—demonstrating "acceptable bias and precision" for the "detection of high concentrations of COHb, found in acute CO poisoning." Values ranged from 0-50% for SpCO and 0-39.3% for COHb with limits of agreement from -3.55% to 9.53% COHb (-4.30% to 7.30% for smokers, -1.63% to 10.29% for nonsmokers). A total of 17 patients (9 smokers, 8 nonsmokers) with a mean COHb of 14.1% received a final diagnosis of CO poisoning. Using a cut-off SpCO value of 6.6%, the researchers found a 94% sensitivity (ability to detect CO poisoning) and 77% specificity (ability to identify a lack of CO poisoning) and stated that the method "appears to be a reasonable upper limit of normal cutoff value for a screening test in the ED setting."
Researchers also found that several factors influence SpCO measurement accuracy as compared to COHb, including smoking, SpCO level, interval between measurements, and age. Because cigarettes increase CO levels in the blood, users were cautioned to pay attention to "the number of cigarettes smoked." Importantly, because CO in the blood decreases with time, researchers cited the "half-life of CO" and "time between multiwave pulse oximetry and blood gas analysis" as other possible "influencing factors on the deviation between SpCO and COHb." The study also shows that time between measurement was still a factor within the 60-minute cut-off established by the researchers—underscoring the importance of simultaneous comparisons between SpCO and invasive blood gas measurements due to the half-life of CO.
Study authors concluded that SpCO measurement via multiwave pulse oximetry has "an acceptable bias and precision" and "keeping influencing factors in mind, it can therefore be used to screen large numbers of patients for latent CO poisoning." They also noted the "potential for different measurement results from correct and incorrect (sensor) placement," an encouragement for users to closely follow the directions for use. The current study follows the results by researchers in the ED of a Rhode Island Hospital, who first demonstrated that large scale population screening of ED patients using SpCO could identify patients with unsuspected CO poisoning.6
1 Roth D, Herkner H, Schreiber W, Hubmann N, Gamper G, Laggner A, Havel C. "Accuracy of Noninvasive Multiwave Pulse Oximetry Compare with Carboxyhemoglobin From Blood Gas Analysis in Unselected Emergency Department Patients." Annals of Emergency Medicine Published April 2011 online ahead of print http://www.annemergmed.com/article/S0196-0644(10)01934-7/abstract.
2 Centers for Disease Control and Prevention. "Nonfatal, unintentional, non–fire-related carbon monoxide exposures—United States, 2004-2006." MMWR Morb Mortal Wkly Rep. 2008;57:896-899.
3 Centers for Disease Control and Prevention. "Carbon monoxide–related deaths—United States, 1999-2004." MMWR Morb Mortal Wkly Rep. 2007;56:1309-1312.
4 Keles A, Demircan A, Kurtoglu G. "Carbon monoxide poisoning: how many patients do we miss?" Eur J Emerg Med. 2008;15:154-157.
5 Hampson NB, Scott KL, Zmaeff JL. "Carboxyhemoglobin measurement by hospitals: implications for the diagnosis of carbon monoxide poisoning." J Emerg Med. 2006;31:13-16.
6 Suner S, Partridge R, Sucov A, et al. "Noninvasive Pulse CO-Oximetry Screening in the Emergency Department Identifies Occult Carbon Monoxide Toxicity." J. Emerg Med. 2008; 34(4): 441-450.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SpCO will provide an accurate and effective method of screening for CO poisoning in large numbers of at-risk populations, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Report First Quarter 2011 Financial Results after Market Close on May 3, 2011
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., April 19, 2011 -- Masimo (NASDAQ: MASI) announced today that it will release first quarter financial results for the period ended April 2, 2011, after the market closes on May 3, 2011.
A conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 58223656. After the live webcast, the call will be available on Masimo's website through June 3, 2011. In addition, a telephonic replay of the call will be available through May 17, 2011. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 58223656.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations,
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


New Published Study Finds Masimo SET® Delivers More Reliable Measurements Significantly Faster Than Other Pulse Oximetry Technologies
Use of Masimo SET Enables Better Adjustments During Critical Neonatal Resuscitation Situations, Aiding in the Prevention of Damage Caused by Unnecessary Exposure to High or Low Oxygen
Irvine, California – April 11, 2011 – Masimo (NASDAQ: MASI) announced today that a new study published in this month's issue of the European peer-reviewed journal Acta Paediatrica demonstrates that pulse oximetry makes a critical difference in neonatal resuscitation both in terms of reliability and speed of measurements. In comparing the measurement response times of three different pulse oximetry technologies, researchers found that the Masimo Radical-7™ pulse oximeter with Masimo SET®Measure-Through Motion and Low Perfusion technology displayed reliable oxygen saturation (SpO2) measurements three to four times faster than competing pulse oximeters.1
Despite numerous advances to improve newborn care, oxygen is still used liberally during newborn resuscitation—unnecessarily exposing many newborns to potentially damaging hyperoxia. Although pulse oximetry is an important clinical tool for evaluating a patient's oxygenation status and guiding resuscitation, measurement failure rates due to motion artifact and low perfusion can be high—leading to inaccurate readings, failure to report readings, or freezing of displayed values. As a result, the time to obtain a reliable oxygen saturation reading during newborn resuscitation in the delivery room and during NICU care is a critically important consideration when choosing a pulse oximetry technology. This is the first prospective observational study to compare pulse oximetry technology performance in detail during newborn resuscitation under unstable critical conditions.
The study was conducted at two health care centers in Barranquilla, Colombia (Clinica del Mar and Medicina Alta Complejidad S.A.) on 32 newborns (median gestational age of 32 weeks) receiving resuscitation as standard of care either in the delivery room or in the Neonatal Intensive Care Unit (NICU). Using the Masimo LNOP®sensor with the Radical-7, the E630 sensor with the Ohmeda Biox 3700, and the OxiMax Max-N sensor for the Nellcor N395, the time to a reliable SpO2 measurement was recorded post-ductally (one sensor placed on each foot) once an adequate pulse rate signal was displayed. Results showed that "the pulse oximeter with Masimo SET provided the fastest response time." In the first comparison of 17 infants, the median response time to obtain a reliable measurement for the Masimo Radical-7 was 20.2±6 (with a range of 18-26 seconds) versus 74.2±12 (with a range of 38-98 seconds) for the Ohmeda Biox 3700. In the second comparison of 15 infants, the median response time for the Masimo Radical-7 was 20.9±4 (with a range of 19-28 seconds) versus 67.3±21 (with a range of 40-90 seconds) for the Nellcor N-395.
Finding that "there are significant differences in the response of pulse oximeters during neonatal resuscitation," researchers concluded that "the speed and reliability of the Masimo SET technology can be of help for clinicians to more accurately adjust the fraction of inspired oxygen during newborn resuscitations, thus preventing or minimizing damage secondary to unnecessary exposure of oxygen and hyperoxemia and to wide fluctuations in oxygen levels."
The 'gold standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® has been shown in over 100 independent clinical studies to provide the most accurate and trustworthy measurements—even under the most challenging clinical conditions, including patient motion and low perfusion.
1 Baquero H, Alviz R, Castillo A, Neira F, Sola A. "Avoiding Hyperoxemia During Neonatal Resuscitation: Time To Response Of Different SpO2 Monitors." Acta Paed April 2011 Vol. 100, Issue 4, pp 515-518. For further reading, visit: http://onlinelibrary.wiley.com/doi/10.1111/j.1651-2227.2010.02097.x/abstract
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SET delivers more reliable measurements significantly faster than other pulse oximetry technologies and the use of Masimo SET enables better adjustments during neonatal resuscitation situations, which may aiding in the prevention of damage caused by unnecessary exposure to high or low oxygen, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Newtech, Inc., a Leading Chinese EMS Patient Monitoring Company, Partners with Masimo to Incorporate rainbow® SET Noninvasive Measurement Capabilities into New Patient Monitors
Shenzhen, China and Irvine, California – March 28, 2011 – Newtech, Inc., and Masimo Corporation (NASDAQ: MASI) jointly announced today a new technology integration strategy that will make the Masimo rainbow SET Pulse CO-Oximetry™ technology platform a fundamental offering in Newtech's emergency medical services (EMS) and multiparameter product line-up—providing clinicians with a host of innovative new noninvasive, continuous measurements and patient monitoring capabilities for the immediate detection of potentially life-threatening conditions.
"By incorporating the Masimo rainbow SET technology into our products, we will be able to offer the EMS market in China the most advanced technologies in monitoring EMS patients," said Ganbing Wang, President of Newtech, Inc. "We see huge potential within China's EMS market over the next five years because of strong government investment to upgrade the EMS system and, being an innovative medical device company, we are pleased to partner with Masimo in providing the best technology for our customers to meet not only their current demands, but their future demands as well."
Masimo rainbow SET noninvasive and continuous measurements—like total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and rainbow Acoustic Monitoring™ (RAM™), in addition to the "gold-standard" Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR)—provide detailed physiological data that helps clinicians to more rapidly assess patients and detect adverse conditions earlier. Immediate availability of such detailed clinical intelligence and measurement data enables clinicians to proactively detect and treat the early signs of hemodynamic instability, potentially life-threatening conditions and internal bleeding sooner rather than reacting to late indicators and their deleterious effects.
Newtech's utilization of broadband wireless technologies allows seamless transmission of patient-critical vital sign information over China's Emergency Medical 3G and 4G networks and the internet. This real-time, live streaming of video, audio and vital signs data provides caregivers with the ability to immediately assess and treat their patients, at the point of care or during ambulance transport. Information is transmitted by the multiparameter handheld (NT1D) or NT3C and NT5 ambulance mounted patient monitors, to a base station, which may be either a remote centralized monitoring system or hospital Emergency Department, where caregivers are alerted in advance and may prepare treatment regimens.
Pre-hospital/EMS early interventions have been shown to dramatically reduce "call to needle" or "door to needle" treatment times, especially in rural areas, resulting in lower mortality rates, improved patient outcomes and survival rates. Incorporating Masimo rainbow SET technology expands the clinician's ability to assess and treat the pre-hospital/EMS patient to now include noninvasive monitoring of critical blood constituents such as hemoglobin (SpHb), Pleth Variability Index (PVI), and carboxyhemoglobin (SpCO), so that a caregiver may evaluate and treat for blood loss, anemia, and even suspected carbon monoxide poisoning before the patient has reached the hospital.
Masimo President of Worldwide OEM Business & Business Development, Rick Fishel, stated, "Masimo's mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. Newtech's innovative approach to real-time monitoring of patients—in remote/rural settings, during ambulance transport, at the point of care, or wherever the care setting may be—represents a shared vision to bring technology to the patient rather than the patient to the technology, so that care may begin as early as possible. We are very pleased that Masimo's unique rainbow Pulse CO-Oximetry platform is a key component in Newtech's new patient monitors."
About Newtech, Inc.
Founded in 1998, Newtech, Inc. is one of the fastest growing medical device manufacturers in China. The company designs, manufactures and markets patient monitoring systems with a current emphasis in EMS monitoring systems. Located in Shenzhen, China, the company was certified by the city government as a "Shenzhen High-tech Enterprise" in 2001. It's quality system was certified to ISO13485 by TüV PS in 2002. The company's products are all SFDA cleared for China market, with many also CE marked or FDA cleared for worldwide distribution. Additional information about Shenzhen Newtech. Inc. and its products is available on the company's website: www.sznewtech.com.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Non-invasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the timing and market availability of Shenzhen Newtech products with Masimo rainbow SET technology, risks related to our assumptions that the integration of Masimo rainbow SET provides advanced noninvasive and continuous measurements and patient monitoring capabilities that have the potential to improve clinical decision-making, outcomes, and patient safety, risks related to our belief that Masimo rainbow SET measurements will enable clinicians to detect potentially life-threatening conditions earlier in all patients and clinical settings, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
For More Information Contact:
Rachel Cheng
NEWTECH, Inc.
650.588.3980
Rachel@solaris-newtech.com
Dana Banks
Masimo Corporation
949.297.7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Non-invasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation. All trademarks and registered trademarks are the sole property of their respective companies.


New Studies Show Clinical Advantages of Masimo Noninvasive Hemoglobin, PVI, and Perfusion Index in Critically-ill Patients
Presented at the International Symposium of Intensive Care and Emergency Medicine (ISICEM) in Belgium
Irvine, California – March 24, 2011 – Masimo (NASDAQ: MASI) announced today that three new studies presented at the International Symposium of Intensive Care and Emergency Medicine (ISICEM) Annual Meeting in Brussels, Belgium,demonstrate the accuracy, reliability, and clinical impact of Masimo noninvasive and continuous measurements—noninvasive hemoglobin (SpHb®), Pleth Variability Index (PVI®), and Perfusion Index (PI)—in Intensive Care Unit (ICU) patients.
"Accuracy of a Continuous Noninvasive Hemoglobin Monitor in Intensive Care Unit"1
Researchers at the Centre Hospitalier Universitaire in Poitiers, France compared the absolute and trend accuracy of Masimo noninvasive hemoglobin (SpHb) to other invasive methods of hemoglobin assessment—including a point of care device (HemoCue301), a satellite lab CO-Oximeter (Siemens RapidPoint 450), and a 'gold-standard' laboratory hematology analyzer (Sysmex XT-2000i)—in 62 critically-ill ICU patients requiring 471 invasive blood samples. Compared to the gold-standard Sysmex hematology analyzer, results showed a bias and limits of agreement for Masimo noninvasive SpHb of 0.0±1.0g/dL. Compared to the gold-standard hematology analyzer, the invasive Hemocue 301 and Rapidpoint 450 had a bias and limits of agreement of 0.3±1.3 g/dL and 0.9±0.6 g/dL, respectively. Citing "absolute and trending accuracy similar to widely used invasive methods," researchers concluded that Masimo SpHb has "the additional advantages of providing continuous measurements, noninvasively, which may facilitate hemoglobin monitoring in the ICU."
"Pleth Variability Index Predicts Fluid Responsiveness in Critically-ill Patients"2
To evaluate whether Masimo PVI, a noninvasive and continuous tool, can help clinicians predict fluid responsiveness in mechanically-ventilated patients with circulatory insufficiency, researchers at the Centre Hospitalier Universitaire in Poitiers, France measured PVI in 40 patients before and after planned volume expansion. Fluid challenge consisted of either 500mL of 130/0.4 hydroxyethyl-starch (fluid) if respiratory variations in arterial pulse pressure > 13%, or with passive leg raising (PLR) otherwise. Results showed 21 patients (19 fluid, 2 PLR) were responders—defined as an increase in cardiac output of >15%. A PVI threshold value of 17% allowed discrimination between responders and non-responders with a sensitivity of 95% (95% confidence interval, 74-100%) and a specificity of 91% (95% confidence interval, 70-99%), leading researchers to conclude that "PVI can predict fluid responsiveness noninvasively in ICU patients under mechanical ventilation." Additionally, PVI at baseline correlated (r=0.72, p<.0001) with changes in cardiac output induced by fluid challenge—suggesting the higher PVI at baseline, the higher the percentage change in cardiac output after volume expansion.
"Perfusion Index as a Predictor for Central Hypovolemia in Humans"3
In this study—the first to evaluate the ability of Masimo PI to detect peripheral vasoconstriction due to neurohumoral response in central hypovolemia-induced by lower body negative pressure (LBNP)—researchers from Erasmus MC University Medical Centre in Rotterdam, Netherlands measured PI in 24 healthy volunteers during LBNP testing. LBNP consisted of 5 min. baseline measurements in the supine position followed by stepwise increases of negative pressure from 0 to -20, -40, -60, -80, and o mmHg. Results showed that PI decreased significantly by 40% (P=0.03) during first -20mmHg and stayed in this range during the remainder of the experiment while stroke volume (SV) decreased by 20% and heart rate (HR) increased by 15% at -40mmHg and were proportional to the level of negative pressure in the chamber. Researchers concluded that PI is a "sensitive indicator of acute hemodynamic responses to LBNP-induced central hypovolemia" and could "detect hypovolemia earlier than 20% decrease in stroke volume."
SpHb, PVI, and PI are available as part of Masimo rainbow®SET platform—the first-and-only technology to noninvasively and continuously measure total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), perfusion index (PI), and acoustic respiration rate (RRa™), in addition to the 'gold standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), and pulse rate (PR).
1 Frasca D, Dahyot-Fizelier C, Karen C. Levrat Q, Soumagne-Vialle N, Boisson M, Debaene B, Mimoz O. "Accuracy of a Continuous Noninvasive Hemoglobin Monitor in Intensive Care Unit." CHU Poitiers, Anesthésie-Réanimation INSERM ERI 23, POITIERS, France. ISICEM Presentation A91.
2 Nanadoumgar H, Loupec TL, Frasca DF, Petitpas FP, Laksiri LL, Baudouin DB, Mimoz OM. "Pleth Variability Index Predicts Fluid Responsiveness in Critically-ill Patients."CHU POITIERS, Reanimation Chirurgicale, Poitiers Cedex, France. ISICEM Presentation A532.
3 Lima A, Van Genderen M, Klijn E, Bartels S, Van Bommel J, Bakker J."Perfusion Index as a Predictor for Central Hypovolemia In Humans." Erasmus MC University Medical Centre Rotterdam, Intensive Care, Rotterdam, Netherlands. ISICEM Presentation A422.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our assumptions that Masimo SpHb accurately tracks and trends Hb changes in all patients, Masimo PVI is accurately predicts fluid responsiveness in all mechanically-ventilated, and Masimo PI is capable of detecting acute LBNP-induced central hypovolemia earlier than stroke volume in all ICU patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at the Roth Growth Stock Conference
IRVINE, Calif., March 3, 2011 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the Roth 23nd Annual Growth Stock Conference at the Ritz-Carlton in Laguna Niguel, Calif. on Tuesday, March 15, 2011, at 9:00 a.m. P.T. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations,
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Royal Fornia Medical Equipment Co., Ltd. Signs Technology Licensing Agreement to Integrate Masimo rainbow SET Pulse CO-Oximetry into New Patient Monitors
One of China's Largest Medical Manufacturers Establishes Pulse CO-Oximetry as the Blood Constituent Monitoring Platform of Choice for New Generation Multiparameter Monitors
Irvine, California & Guangdong Province, China – February 24, 2011 – Masimo (NASDAQ: MASI) and Royal Fornia Medical Equipment Co., Ltd. jointly announce a worldwide technology licensing agreement to integrate Masimo rainbow SET®Pulse CO-Oximetry™ technology into Royal Fornia's new generation of multiparameter patient monitors—enabling advanced noninvasive blood constituent measurements that help clinicians to detect and treat life-threatening conditions earlier. Having recently received State Food and Drug Administration (SFDA) approval for Masimo rainbow®SET parameters in China, Royal Fornia establishes itself as one of the first domestic Chinese medical device manufacturers to commercially incorporate Masimo's breakthrough noninvasive blood constituent measurement capabilities.
Masimo rainbow®SET noninvasive and continuous measurements—like total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to the 'gold-standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR)—provide detailed physiological data that helps clinicians to more rapidly assess patients and detect adverse conditions much earlier. With rainbow®SET measurements, the ability to noninvasively detect internal bleeding, carbon monoxide poisoning, methemoglobinemia, fluid responsiveness, low perfusion, desaturations, and abnormal pulse rates quickly, easily, and continuously can help save lives and improve patient outcomes. Immediate availability of such detailed clinical intelligence and measurement data enables clinicians to proactively detect and treat the early signs of hemodynamic instability, potentially life-threatening conditions, and bleeding sooner rather than reacting to late indicators and their deleterious effects.
According to Mr. Li Yang, Vice President of Royal Fornia Medical Equipment Co., Ltd, "Our corporate mission is to provide innovative medical devices with superior performance and value. The integration of Masimo rainbow®SET technology will allow us to design and manufacture state-of-the-art patient monitors that advance the delivery of healthcare. We expect that our combined product offering—Masimo's rainbow®SET measurements within our JCY-800-I and JCY-800-III multiparameter monitors—will provide clinicians with critical access to the latest cutting-edge technologies that allow them to more thoroughly assess a patient's physiological status and make better clinical and treatment decisions across the continuum of care."
Masimo President of Worldwide OEM Business and Corporate Development, Rick Fishel, stated, "Royal Fornia's 'first-to-market' commitment and strategy provides its customers with easy access to an upgradeable platform of blood constituent monitoring parameters that provide new clinical intelligence and detailed physiological data to improve patient safety and care while optimizing reimbursement potential—all consolidated in their new portable line of multiparameter patient monitors. Royal Fornia has a history of developing innovative technology solutions to address previously unmet clinical needs, and we are very pleased that Masimo's unique rainbow®Pulse CO-Oximetry platform is a key component in their new portable patient monitors. "
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
About Royal Fornia Medical Equipment Co., Ltd.
As a China-US joint venture, Royal Fornia Medical Equipment Co., Ltd. was founded in 2000 and is engaged in R&D, manufacturing and distributing of high-end medical electronic devices. Now, it has comprehensive sales network and well-developed after-sales service, which further facilitate and improve the promotion of its products. We are a group of professionals who follow the concept of "Human-oriented technology" and it contributes a lot to Royal Fornia's rapid growth. Under the guidance of our business philosophy "Health Care for Better Future," Royal Fornia will always pursue the advanced technologies and outstanding product quality, and will devote itself to the making of an excellent corporation in the high-tech industry. For more information visit: www.rfornia.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that Masimo rainbow SET Pulse CO-Oximetry provides detailed physiological data that helps clinicians to more rapidly assess all patients and detect adverse conditions much earlier; risks related to our belief that rainbow measurements can noninvasively detect internal bleeding, carbon monoxide poisoning, methemoglobinemia, fluid responsiveness, low perfusion, desaturations, and abnormal pulse rates quickly, easily, and continuously for all patients; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, Pulse CO-Oximetry, Pulse CO-Oximeter, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57, Rad-8, Rad-5, Pronto-7, Pronto, Patient SafetyNet, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at the Cowen and Company Health Care Conference
IRVINE, Calif., February 22, 2011 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the Cowen and Company Health Care Conference at The Marriott Copley Place in Boston on Tuesday, March 8, 2011, at 8:45 a.m. E.T. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations,
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation


Mater Children's Hospital in Australia Converts to Masimo rainbow SET for State-of-the-Art Noninvasive Patient Monitoring Capabilities
Queensland's Leading Pediatric Hospital Standardizes to Pulse CO-Oximetry—Offering Advanced Monitoring of Blood and Multiple Other Physiological Parameters Without Invasive Procedures
Irvine, California & Queensland, Australia – February 21, 2011 – Mater Children's HospitalandMasimo (NASDAQ: MASI) today jointly announce the conversion of Mater Children's Hospital to Masimo rainbow®SET Pulse CO-Oximetry™ technology for advanced noninvasive patient monitoring capabilities. The conversion equips the tertiary facility with the most technologically and clinically-advanced oximetry and noninvasive patient monitoring solutions available.
Masimo rainbow®SET Pulse CO-Oximetry is the first-and-only technology platform that allows hospitals to noninvasively measure and continuously monitor blood constituents and physiological parameters that previously required invasive procedures—including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the 'gold-standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR). The oximetry standard-of-care at leading hospitals worldwide, Masimo rainbow®SET provides immediate real-time results that enable clinicians to more rapidly assess patients and detect and treat adverse, potentially life-threatening conditions earlier.
"Converting to Masimo rainbow®SET provides our clinicians with superior patient monitoring capabilities and advanced clinical measurements thereby ensuring that our clinicians have access to quality clinical data," stated Malcolm Thatcher, Chief Information Officer for Mater Health Services. "The decision to standardize on Masimo's technology platform across our pediatric services was driven by the knowledge that, in harnessing the advanced noninvasive capabilities of Masimo rainbow®SET measurements, Mater is able to deliver an exceptional level of care and safety to our pediatric patients while recognizing the importance of the patient experience and supporting our organization's commitment to putting the patient first."
A major referral center for specialty pediatric healthcare services in Queensland, Mater Children's Hospital provides a range of inpatient, outpatient, and emergency care to over 167,000 children a year. The conversion ensures that all multiparameter patient monitors, stand-alone oximeters, and sensors feature the advanced noninvasive patient monitoring capabilities of Masimo rainbow®SET—allowing clinicians to easily and painlessly obtain and continuously track vital blood and physiological data for patients in real-time, anywhere in the hospital.
Mater Children's Hospital Pediatric Sleep Scientist, Gordon Williams, said the Sleep Unit relies on Masimo SET's ability to measure oxygen saturation during movement, which he says is "especially important in a pediatric facility where motion-artifact can be a major source of oximeter signal noise. Not only does Masimo's technology allow us to obtain more reliable saturation trend data in children being investigated in the sleep laboratory, but we are able to confidently use the Masimo Radical-7 Pulse CO-Oximeter as a screening and assessment tool on the wards as well. And in terms of future needs, Masimo's rainbow®SET Pulse CO-Oximetry platform positions us for growth well beyond how oximeters have traditionally been used to date."
Masimo Founder and CEO, Joe Kiani, stated, "Reducing the trauma of invasive tests and procedures for pediatric patients has long been a concern for every clinician and parent that cares for a sick child. Today, thanks to medical advancements enabled by Masimo rainbow®SET Pulse CO-Oximetry and the forward-thinking efforts of Mater Children's Hospital, pain-free hemoglobin testing and clinically reliable pulse oximetry is now a reality for pediatric patients in Queensland."
About Mater Children's Hospital
Mater Children's Hospital is a major tertiary pediatric referral centre with the busiest pediatric emergency department in Queensland. The hospital provides specialty healthcare services to more than 15,000 inpatient, 120,000 outpatient, and 32,000 emergency patients each year. To learn more about Mater, visit www.mater.org.au/Home/Hospitals/Mater-Children-s-Hospital.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the hospital-wide conversion ensures that all Mater Children's Hospital patients will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo rainbow SET provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient; risks related to our belief that SpHb detects low or falling hemoglobin levels that could be the result of internal bleeding; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Brooke Falvey
Mater Children's Hospital
(07) 3163 3495
brooke.falvey@mater.org.au
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, Pulse CO-Oximetry, Pulse CO-Oximeter, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57, Rad-8, Rad-5, Pronto-7, Pronto, Patient SafetyNet, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Reports Fourth Quarter and Full Year 2010 Financial Results; Provides 2011 Guidance
Q4 2010 Highlights (compared to Q4 2009):
- Total revenue rose 14.0% to $105.6 million
- Product revenue rose 16.6% to $93.8 million
- Masimo SET and Masimo rainbow SET unit shipments rose 37.5% to 41,800
- Masimo rainbow revenue rose 44.4% to $8.4 million
- GAAP EPS rose 13.0% to $0.26. Excluding one-time expenses, adjusted EPS rose 26.1% to $0.29.
Full Year 2010 Highlights (compared to 2009)
- Total revenue rose 16.1% to $405.4 million
- Product revenue rose 18.8% to $356.4 million
- Masimo SET and Masimo rainbow SET unit shipments rose 37.5% to 153,200
- Masimo rainbow revenue rose 69.0% to $32.9 million
- GAAP EPS rose 37.5% to $1.21. Excluding one-time items, adjusted EPS rose 17.1% to $1.03.
Irvine, California, February 15, 2011 – Masimo (NASDAQ: MASI) today announced its financial results for the fourth quarter and year ended January 1, 2011.
Masimo's total revenue for the fourth quarter rose 14.0% to $105.6 million, compared to $92.6 million for the fourth quarter of 2009. Masimo's fourth quarter product revenue rose 16.6% to $93.8 million, compared to $80.5 million for the fourth quarter of 2009. Revenue from Masimo rainbow products rose 44.4% to $8.4 million in the fourth quarter, compared to $5.8 million for the fourth quarter of 2009.
Net income for the fourth quarter was $16.1 million, or $0.26 per diluted share, including $0.02 per diluted share in one-time marketing and other related expenses that Masimo had previously planned and announced after receiving $30.8 million in proceeds in 2010 from an antitrust lawsuit against Covidien. Excluding these one-time marketing and other related expenses, adjusted net income for the fourth quarter was $17.5 million, or $0.29 per diluted share, compared to net income of $14.1 million, or $0.23 per diluted share, in the fourth quarter of 2009. Due to rounding, the impact of excluding $0.02 per diluted share in one-time marketing and other related expenses added $0.03 per diluted share to the adjusted earnings per share calculation in the fourth quarter of 2010.
For 2010, Masimo's total revenue rose 16.1% to $405.4 million, compared to $349.1 million for 2009. The company's product revenue rose 18.8% in 2010 to $356.4 million, compared to $300.1 million in 2009. Revenue from Masimo rainbow products rose 69.0% to $32.9 million in 2010, compared to $19.5 million in 2009.
Masimo's net income for 2010 was $73.5 million, or $1.21 per diluted share, including $0.18 per diluted share from the net of a one-time gain related to the $30.8 million in antitrust proceeds from Covidien and offset by $14.7 million in marketing and other related expenses that Masimo had previously planned and announced after receiving the proceeds. Excluding these one-time items, adjusted net income for 2010 was $62.5 million, or $1.03 per diluted share, compared to net income of $53.2 million, or $0.88 per diluted share, in 2009.
During the fourth quarter, the company shipped approximately 41,800 Masimo SET pulse oximetry and Masimo rainbow SET Pulse CO-Oximetry units, excluding handheld units, up 37.5% compared to approximately 30,400 in the same prior year period. For 2010, Masimo shipped approximately 153,200 Masimo SET pulse oximetry and Masimo rainbow SET Pulse CO-Oximetry units, excluding handheld units, up 37.5% compared to approximately 111,400 in 2009. Masimo estimates its worldwide installed base as of January 1, 2011 to be 855,000 units, up 18.0% from 724,000 units as of January 2, 2010.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "Our strong fourth quarter and fiscal 2010 performance was driven by increasing global demand for our breakthrough Masimo SET and Masimo rainbow SET technologies, allowing us to advance our mission to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications."
As of January 1, 2011, cash, cash equivalents and short-term investments totaled $88.3 million, compared to $189.0 million as of January 2, 2010. The decline was due primarily to special dividend payments of $117.5 million and $44.5 million, made on March 31, 2010 and December 21, 2010, respectively. Total 2010 dividend payments of $162.0 million were partially offset by the net proceeds from the Covidien antitrust lawsuit and operating cash flow during the year.
2011 Financial Guidance
Masimo expects fiscal 2011 total revenue to be between $446 million and $463 million, including product revenue in the range of $415 million to $430 million, and royalty revenue in the range of $31 million and $33 million. Included within these product revenue ranges are 2011 rainbow revenue expectations of $40 million to $50 million. As a result, the company expects fiscal 2011 GAAP earnings per share to be between $1.17 and $1.25. Each of the components of Masimo's guidance set forth above is an estimate only and actual performance could differ.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 679-9937 for international callers. The reservation code for both dial-in numbers is 38781834. After the live webcast, the call will be available on Masimo's website through March 15, 2011. In addition, a telephonic replay of the call will be available through March 1, 2011. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 38781834.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies; and expectations for total revenue, royalty revenue and product revenue, including rainbow revenue, and GAAP earnings per share for the full fiscal year 2011. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations,
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.





Mafraq Hospital Becomes First in United Arab Emirates to Deploy Masimo rainbow SET Pulse CO-Oximetry for State-of-the-Art Noninvasive Patient Monitoring
Largest Hospital in the UAE Converts to Pulse CO-Oximetry Technology—Offering Advanced Monitoring of Blood Constituents and Multiple Physiological Parameters Without Invasive Procedures
Irvine, California and Dubai, UAE – February 10, 2011 – Mafraq Hospital andMasimo (NASDAQ: MASI) jointly announced the hospital-wide conversion of Mafraq Hospital to Masimo rainbow®SET Pulse CO-Oximetry™ technology for advanced noninvasive patient monitoring capabilities. The conversion makes Mafraq Hospital the first hospital in the UAE to feature Masimo's technologically and clinically-advanced oximetry and noninvasive patient monitoring solutions.
The Masimo rainbow SET Pulse CO-Oximetry technology platform allows hospitals to noninvasively measure and continuously monitor multiple blood constituents and physiological parameters that previously required invasive procedures—including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the 'gold-standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR). The oximetry standard-of-care at leading hospitals worldwide, Masimo rainbow SET provides immediate real-time results that enable clinicians to more rapidly assess patients and detect and treat adverse, potentially life-threatening conditions earlier.
"Mafraq Hospital takes great pride in the skills and experience of its physicians and the fact that we have some of the world's most advanced equipment available, so that we can provide the best medical care and treatment services in one of the fastest growing areas in the Emirate of Abu Dhabi," stated John Nickens, CEO at Mafraq Hospital. "We saw tremendous value in the superior performance and futuristic noninvasive capabilities of Masimo rainbow SET Pulse CO-Oximetry and wanted to extend its positive clinical impact and patient care benefits to all of our patients throughout the hospital."
Mafraq Hospital, managed by Bumrungrad International and owned and operated by the Abu Dhabi Health Services Company PJSC (SEHA), which is responsible for all the curative activities of public hospitals and clinics in Abu Dhabi, is a major referral hospital and comprehensive tertiary healthcare provider in the UAE for 510,000 patients each year. The conversion ensures that all multiparameter patient monitors, stand-alone oximeters, and sensors throughout the hospital will feature the advanced noninvasive patient monitoring capabilities of Masimo rainbow SET—allowing Mafraq Hospital clinicians to easily and painlessly obtain and continuously track vital blood and physiological data for patients in real-time. Mafraq Hospital initially launched the new technology platform hospital-wide with Masimo SET pulse oximetry measurements (SpO2, PI, and PR), enabling the hospital to quickly and easily upgrade to Masimo rainbow Pulse CO-Oximetry measurements (SpHb, SpOC, PVI, SpCO, SpMet) as needed.
According to Mafraq Hospital Chief Nursing Officer, Gail Smith, "This conversion provides an exciting new medical first—noninvasive and continuous hemoglobin (SpHb), which allows our clinicians to perform trauma-free hemoglobin blood tests and get immediate results. And, as the only hospital in the UAE equipped with SpHb, we not only have the ability to quickly measure hemoglobin levels without removing a drop of blood from the patient, but we can now continuously track hemoglobin levels in real-time without having to perform repeated, painful blood draws and time-consuming lab analyses."
Masimo Founder and CEO, Joe Kiani, stated, "We are happy to be partnering with Mafraq Hospital as they lead the UAE in advancing the care and safety of patients. Mafraq Hospital's adoption of Masimo rainbow SET now makes it possible for hospital clinicians to noninvasively monitor and continuously track key indicators of a patient's relative physiological status—including hemoglobin and other blood constituents as well as fluid volume and respiration rate—to immediately and painlessly identify abnormalities and detect potentially life-threatening conditions much earlier."
The CEO of Emitac Healthcare Solutions (EHS), Masimo's distributor in the UAE, Mr. Raghavan Srinivasan considers it a matter of pride to partner with Masimo in deploying this solution to Mafraq Hospital, stating that: "This is a progressive step by the hospital to adopt innovative means to providing quality healthcare in the country."
About Mafraq Hospital
Mafraq Hospital was established in 1983 and is one of the largest tertiary referral treatment hospitals in the United Arab Emirates, with 451 licensed beds. Its specialist services include medicine, obstetrics, pediatrics, as well as surgical and critical care services. Mafraq Hospital operates the largest burn unit in the country and is a Center of Excellence for ENT and Thoracic surgery. The hospital also operates two primary healthcare clinics at Bani Yas and Al Nahda. Mafraq Hospital is part of the SEHA Health System and is owned and operated by Abu Dhabi Health Services Company PJSC (SEHA) which is responsible for the curative activities of the public hospitals and clinics of the Emirate of Abu Dhabi. It is managed by Bumrungrad International (BIL), one of the most noted hospital management companies in Asia. Mafraq Hospital physicians are amongst the best in their specialist fields with international qualifications and world class expertise. The medical staff is supported by a capable and professional nursing and paramedical staff who are well trained and motivated to provide quality healthcare services. Emiratization is extremely important to Mafraq Hospital and many of the hospital's highly skilled physicians are UAE Nationals. The hospital also believes in customer service excellence and in providing quality healthcare with integrity and service excellence in a caring environment.
About the SEHA Health System and the Abu Dhabi Health Services Company PJSC (SEHA)
SEHA is health in Arabic. The Abu Dhabi Health Services Company PJSC – whose marketing identity is SEHA – is an independent, public joint stock company created to develop the curative activities of the public healthcare system in Abu Dhabi. The company owns and operates all the public hospitals and clinics of the Emirate of Abu Dhabi which together make up the SEHA Health System. SEHA is committed to providing high quality, cost effective healthcare in a socially responsible way on par with international standards measured through accessibility, affordability, choice and satisfaction. SEHA has partnered with internationally recognized hospital managers to achieve these goals. These include Johns Hopkins Medicine International, Cleveland Clinic Foundation, VAMED, Vienna Medical University and Bumrungrad International. SEHA owns and operates 12 hospitals with 2,644 beds, 62 ambulatory care, family care and urgent care centers and 2 blood banks. SEHA is one of the largest integrated healthcare providers in the Middle East with 16,500 doctors, nurses, ancillary care and administrative personnel in its employ. For more information, visit www.seha.ae.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the hospital-wide conversion ensures that all Mafraq patients will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo rainbow SET provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient; risks related to our belief that SpHb detects low or falling hemoglobin levels that could be the result of internal bleeding; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Amanda Banham
Mafraq Hospital
+971 2 5012443
abanham@mafraqhospital.ae
Dana Banks
Masimo Corporation
+1(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, Pulse CO-Oximetry, Pulse CO-Oximeter, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57, Rad-8, Rad-5, Pronto-7, Pronto, Patient SafetyNet, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Welch Allyn Establishes Strategic Partnership to Incorporate Masimo rainbow® SET Noninvasive Measurement Capabilities in Next-Generation Patient Monitors
Company will integrate Masimo's advanced Pulse CO-Oximetry™ technology into its new Connex®Vital Signs Monitor and future product releases
Skaneateles Falls, New York and Irvine, California – February 8, 2011 – Welch Allyn and Masimo (NASDAQ: MASI) jointly announced today a new technology integration strategy that will make the Masimo rainbow®SET Pulse CO-Oximetry™ technology platform a strategic and fundamental offering in Welch Allyn's new product line-up—providing clinicians with a host of innovative new noninvasive, continuous measurements and patient monitoring capabilities. In addition, the unique upgradeability inherent within the rainbow SET technology platform, coupled with the flexibility and configurability of next-generation Welch Allyn patient monitors, will allow clinicians the option to remotely add Masimo's noninvasive and continuous measurements as optional software upgrades as the measurements become commercially available for use with the Welch Allyn patient monitor.
"Welch Allyn is excited to provide our customers with the option of enabling the Masimo parameters within our patient monitoring devices in a manner that considers their evolving needs over time," said Welch Allyn Executive Vice President and Chief Global Business Officer, Steve Meyer. "Masimo technology supports our platform concept of modularity and upgradeability while allowing customers to improve patient care through early, noninvasive continuous and spot check monitoring of key hemodynamic parameters."
Masimo rainbow SET noninvasive and continuous measurements—like total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and rainbow Acoustic Monitoring™ (RAM), in addition to the "gold-standard" Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR)—provide detailed physiological data that helps clinicians to more rapidly assess patients and detect adverse conditions earlier. Pending regulatory clearance, Welch Allyn will announce the commercial availability of these rainbow SET parameters as they become legally available for use with its monitors.
Immediate availability of such detailed clinical intelligence and measurement data enables clinicians to proactively detect and treat the early signs of hemodynamic instability, potentially life-threatening conditions and internal bleeding sooner rather than reacting to late indicators and their deleterious effects.
The new Welch Allyn Connex®Vital Signs Monitor (CVSM) is the first Welch Allyn monitor to integrate the Masimo rainbow SET technology. The CVSM, which launched in August 2010, incorporates Masimo's SpO2-enabled measurements and is capable of being field upgraded to add rainbow SET-based measurements as each parameter becomes commercially available for use with the CVSM.
The CVSM is a full-color, touch screen device that acts as three devices in one—providing comprehensive patient documentation on a single display. This documentation includes automatic measurements such as heart rate, blood pressure, temperature and pulse oximetry; manual parameters such as respiration, height, weight and pain level; and modifiers such as body position, O2 therapy details and others. The device also gives the clinician the ability to control alarms, patient data and monitoring in a customized manner for each patient, and document the data right on the device—eliminating the need to locate a PC and transcribe it later.
Welch Allyn will offer customers the ability to remotely upgrade the device's software to add additional Masimo rainbow SET capabilities via the Web using the company's innovative PartnerConnect™ Remote Services solution. PartnerConnect is a feature of the Welch Allyn Partners in Care ServicesSM program that enables remote diagnostics of devices and systems. Software installations, updates, and upgrades can all be delivered and performed remotely. PartnerConnect also enables preemptive service capabilities on the Welch Allyn platform, allowing the Partners in Care Technical Support Center to remotely view devices and system configurations in real time via the Internet.
Masimo President of Worldwide OEM Business, Business Development, and Masimo Americas, Rick Fishel, stated, "Integrating industry-leading noninvasive and continuous monitoring technologies that offer point-of-care, cost-effective measurements and documentation is a growing trend in both continuous and spot-check patient monitors. The Welch Allyn Connex Vital Signs Monitor represents an innovative monitoring platform in many ways, including its point-of-care configurability, upgradeability, wireless EHR connectivity, and next-generation patient monitoring capabilities—all of which help clinicians meet the patient care challenges and clinical demands of today and tomorrow. We are proud of the positive impact our medical technology innovations have on patient care, safety, and clinical outcomes, and we are equally proud to be a key part of Welch Allyn's forward-looking patient monitoring technology strategy."
About Welch Allyn
Founded in 1915 and headquartered in Skaneateles Falls, NY (USA), Welch Allyn is a leading global provider of medical diagnostic equipment and a complete range of digital and connected solutions. With 2,600 employees working in 26 different countries, Welch Allyn specializes in helping doctors, nurses, and other frontline practitioners across the globe provide the best patient care by developing innovative products, breakthrough technologies, and cutting- edge solutions that help them see more patients, detect more conditions, and improve more lives. More information about Welch Allyn and its complete line of connected products and solutions may be found at www.welchallyn.com
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the timing and market availability of Welch Allyn products with Masimo rainbow SET technology, risks related to our assumptions that the integration of Masimo rainbow SET provides advanced noninvasive and continuous measurements and patient monitoring capabilities that have the potential to improve clinical decision-making, outcomes, and patient safety, risks related to our belief that Masimo rainbow SET measurements will enable clinicians to detect potentially life-threatening conditions earlier in all patients and clinical settings, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Jamie Arnold, APR
Public Relations Manager
Welch Allyn
315-685-4599
Dana Banks
Manager, Public Relations
Masimo Corporation
949-297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5, Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


New Published Study Finds Noninvasive Masimo PVI Accurately Predicts Fluid Responsiveness in Intensive Care Patients
PVI Predictive Ability Similar to Invasive Pulse Pressure Variation and Superior to Cardiac Output
Irvine, California – February 1, 2011 – Masimo (NASDAQ: MASI) announced today that a new study published in the peer-reviewed journal Critical Care Medicine demonstrates that noninvasive, continuous monitoring of Masimo Pleth Variability Index (PVI®) predicts fluid responsiveness with high sensitivity (95%) and specificity (91%) in mechanically-ventilated patients in the Intensive Care Unit (ICU).1 Multiple previous studies have shown that PVI predicts fluid responsiveness in surgical patients—helping clinicians improve fluid management to reduce patient risk,2,3,4 but this is the first published study demonstrating its effectiveness in predicting fluid responsiveness in critically-ill ICU patients.
Hemodynamic instabilityis a common problem for critically-ill patients, but the decision to administer fluid in an attempt to improve cardiac output is challenging. When necessary, fluid administration is critical to optimizing patient status and enabling end organ preservation, but unnecessary fluid administration is associated with increased morbidity and mortality. However, other dynamic indicators for assessing intravascular fluid volume are either invasive, operator dependent, costly, or unreliable predictors of whether a patient will benefit from fluid administration.
Citing numerous prospective studies showing that "only half of critically-ill patients respond to fluid boluses deemed necessary by attending physicians," researchers from CHU de Poitiers (France) acknowledged the "need for simple, noninvasive, and continuous bedside monitoring capable of detecting fluid responsiveness early in virtually all ICU patients, including those without an arterial catheter" in undertaking the current study. PVI provides clinicians with a noninvasive, continuous, and cost-effective measure for assessing whether patients will benefit from fluid administration—enabling clinicians to provide personalized and goal-directed fluid therapy1,2,3,4.
To evaluate the predictive ability of fluid responsiveness indicators, study researchers compared three indices in 40 mechanically-ventilated patients with circulatory insufficiency in the ICU. The indices ranged in level of difficulty to perform—from automatic (PVI, noninvasively obtained via the Masimo Radical-7 Pulse CO-Oximeter) to invasive (∆PP, respiratory variations in arterial pulse pressure obtained via arterial catheter) to operator-dependent (CO, cardiac output obtained via echocardiography). Each of the indices was recorded before and after fluid challenge, which consisted of either 500mL of 130/0.4 hydroxyethyl-starch (fluid) infused over 10 minutes with the patient in a semi-recumbent position (head at 45 degrees) or passive leg raising (PLR) if ∆PP <13%—indicating that the patient would be unlikely to respond to fluid administration.
Results showed 21 patients (19 fluid, 2 PLR) were responders—defined as an increase in cardiac output of >15% after fluid or PLR—and 19 patients (2 fluid, 17 PLR) were non-responders. A threshold value of 17% for PVI allowed discrimination between responders and non-responders at 95% sensitivity and 91% specificity and a ∆PP threshold value of 10% allowed discrimination between responders and non-responders at 100% sensitivity and 95% specificity. However, cardiac output (CO) was unable to reliably predict responders vs. non-responders. Additionally, responders had significantly higher average values vs. non-responders for PVI (28% vs. 11%) and ∆PP (22% vs. 5%) and both PVI (r=0.72, p<.0001) and ∆PP (r=0.77, p<.0001) correlated with the percentage change in CO induced by the fluid challenge—suggesting that "the higher PVI or ∆PP is at baseline, the higher the beneficial effect of volume expansion."
Finding that the "performance of PVI to predict fluid responsiveness was similar to that of ∆PP," researchers concluded that Masimo PVI "can predict fluid responsiveness in intensive care unit patients under mechanical ventilations." Researchers also noted the implications of using such dynamic fluid responsiveness predictors, commenting that "optimizing the patient's hemodynamic state with ∆PP or PVI monitoring has potential to decrease duration of hospital stay and mechanical ventilation, postoperative morbidity, and costs in patients undergoing high-risk surgery."
PVI is available as part of Masimo rainbow®SET platform—the first-and-only technology to noninvasively and continuously measure total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), perfusion index (PI), and acoustic respiration rate (RRa™), in addition to the 'gold standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), and pulse rate (PR).
1 Loupec T., Nandoumgar H., Frasca D., Petitpas F., Laksiri L., Baudouin D., Debaene B., Dahyot-Fizelier C., Mimoz O. "Pleth Variability Index Predicts Fluid Responsiveness in Critically-Ill Patients." Crit Care Med February 2011 Vol. 39, Issue 2, pp 294-299. For further reading, visit: http://journals.lww.com/ccmjournal/Abstract/2011/02000/Pleth_variability_index_predicts_fluid.8.aspx.
2 Cannesson M., Desebbe O., Rosamel P., Delannoy B., Robin J., Bastien O., Lehot JJ. "Pleth variability Index to Monitor the Respiratory Variations in the Pulse Oximeter Plethysmographic Waveform Amplitude and Predict Fluid Responsiveness in the Operating Theatre." British Journal of Anaesthesia August 2008; 101(2):200-6. For further reading, visit: http://www.ncbi.nlm.nih.gov/pubmed/18522935.
3 Zimmerman M., Feibicke T., Keyl C., Prasser C., Moritz S., Graf B., and Wiesenack C. "Accuracy of Stroke Volume Variation Compared with Pleth Variability Index to Predict Fluid Responsiveness in Mechanically-ventilated Patients Undergoing Major Surgery." European Journal of Anaesthesiology June 2010; 27(6):555-61. For further reading, visit: http://www.ncbi.nlm.nih.gov/pubmed/20035228.
4 Feissel M., Kalakhy R., Badie J., Robles G., Faller J., Teboul JL. "Plethysmography Variability Index: A New Fluid Responsiveness Parameter." Presented at the 29th International Symposium on Intensive Care and Emergency Medicine (ISICEM) Annual Meeting, March 25, 2009, Brussels, Belgium. For further reading, visit: http://ccforum.com/content/13/S1/P205.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo PVI is capable of detecting fluid responsiveness in virtually all ICU patients, and risks related to our assumptions that Masimo PVI monitoring has the potential to decrease hospital stay, mechanical ventilation, postoperative morbidity, and costs in patients undergoing high-risk surgery, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Provides Additional Comments Regarding Amendment to Settlement Agreement with Covidien
IRVINE, Calif., January 31, 2011 – Following today's announcement by Masimo (NASDAQ: MASI) that the company has amended its January 2006 settlement agreement with Covidien, Masimo provides the following additional information.
In conjunction with finalizing its 2010 financial results, Masimo is currently assessing the full financial impact of this amendment on its 2011 financial outlook. As part of this assessment, Masimo is considering the possibility of re-investing up to 50% of this incremental royalty revenue into its business in 2011. Masimo intends to provide 2011 guidance and discuss the impact of the amendment when the company announces its 2010 financial results.
Masimo announced last Friday that it will discuss its fourth quarter and full year 2010 financial results in a conference call and webcast on Tuesday, February 15, 2011, at 1:30 p.m. PT (4:30 p.m. ET).
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic MonitoringTM, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our amended royalty agreement. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce the anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations,
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.

Masimo and Covidien Announce Extension of Royalty Agreement
IRVINE, Calif., January 31, 2011 – Masimo (NASDAQ: MASI) and Covidien today announced that they have amended the existing settlement agreement initially reached in January 2006. Under the terms of the amendment, Covidien will pay Masimo a royalty of 7.75% for its current pulse oximetry products sold in the United States for three years, beginning March 15, 2011.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our amended royalty agreement. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce the anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations,
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Report Fourth Quarter and Full Year 2010 Financial Results after Market Close on February 15, 2011
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., January 28, 2011 -- Masimo (NASDAQ: MASI) announced today that it will release fourth quarter and full year 2010 financial results for the period ended January 1, 2011, after the market closes on February 15, 2011.
A conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 679-9937 for international callers. The reservation code for both dial-in numbers is 38781834. After the live webcast, the call will be available on Masimo's website through March 15, 2011. In addition, a telephonic replay of the call will be available through March 1, 2011. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 38781834.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations,
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


New Clinical Study Finds Masimo Noninvasive Hemoglobin Accurate in Patients with Critically Low Hemoglobin Levels
Study Presented at Society of Critical Care Medicine Demonstrates Masimo SpHb's Potential to Improve Blood Management Decisions in Severely Anemic Intensive Care Patients
Irvine, California – January 20, 2011 – Masimo (NASDAQ: MASI) announced today that a new study demonstrating the clinical accuracy and value of its noninvasive and continuous hemoglobin (SpHb®) monitoring technology breakthrough was presented this week at the Society of Critical Care Medicine (SCCM) Annual Critical Care Congress in San Diego. The largest multi-professional critical care event of the year, the SCCM Annual Congress reflects the latest in evidence-based research, clinical best practices, and medical developments that are shaping the future of critical care medicine.
The study, presented by researchers at Englewood Hospital and Medical Center (EHMC) in New Jersey, evaluated the accuracy of Masimo SpHb — a noninvasive measurement of hemoglobin blood levels obtained using a Masimo rainbow®ReSposable Sensor placed on the finger and continuously displayed on a Masimo Radical-7®Pulse CO-Oximeter™.SpHb measurements obtained from nine intensive care unit (ICU) patients with critically low hemoglobin levels (ranging from 4.3-8.6g/dL for Hb lab values) were compared with invasive blood samples drawn simultaneously and analyzed by a central laboratory. Results showed a mean bias and precision of 0.70 g/dL and 1.05 g/dL for SpHb, respectively, when compared to reference laboratory hemoglobin values—demonstrating "clinically acceptable agreement." Researchers concluded that "the ability to measure hemoglobin noninvasively and continuously has the potential to facilitate the timely detection of changes in hemoglobin and thus improve patient blood management decisions in patients with critically low hemoglobin."1
According to lead researcher, Anna Juhl, RN, Clinical Research Director of Anesthesiology and Critical Care at EHMC, "Two important elements of Patient Blood Management (PBM) are: 1) managing post-op blood loss with any resulting anemia, and 2) only transfusing based on clinical and lab guided evidence. The results of this study show that Masimo SpHb may help aid clinicians in achieving the goals of PBM in a timely fashion with less blood loss to the patient."
According to Dr. Michael O'Reilly, Chief Medical Officer at Masimo, "Successfully managing patients with very low hemoglobin levels is often a complex and risky undertaking that most physicians avoid by giving blood transfusions. However, the growing significance of deleterious transfusion effects and complications are now forcing physicians to re-evaluate transfusion decisions in critically ill patients. This study showcases Masimo SpHb's importance and unique role in aiding the clinical management of patients with very low hemoglobin levels—providing clinicians with a new tool to monitor and manage very low hemoglobin levels more effectively."
SpHb is available as part of the Masimo rainbow®SET platform—the first-and-only technology to noninvasively and continuously measure total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the 'gold-standard' Measure-Through Motion and Low Perfusion performance of Masimo SET®oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR).
1 Juhl A., Naqvi S., Aregbeyen O., Demir S., Shander A. "Accuracy of Noninvasive Hemoglobin Monitoring in Intensive Care Unit Patients with Very Low Hemoglobin." Englewood Hospital and Medical Center, Englewood, New Jersey. SCCM abstract #215 presented January 19, 2011.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, Pulse CO-Oximetry, Pulse CO-Oximeter, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57, Rad-8, Rad-5, Pronto-7, Pronto, Patient SafetyNet, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


New Johns Hopkins School of Medicine Study Shows Masimo Noninvasive Hemoglobin Monitoring is Accurate During Complex Spine Surgeries
Study Presented at Society for Technology in Anesthesia Demonstrates Masimo SpHb May Enable More Rapid Assessments of Patient Condition with Potentially Improved Blood Management Decisions
Irvine, California – January 18, 2011 – Masimo (NASDAQ: MASI) announced today that a new clinical study demonstrating the clinical accuracy of its noninvasive and continuous hemoglobin (SpHb®) monitoring technology breakthrough was presented last week at the Society for Technology in Anesthesia (STA) Annual Meeting.
The study, presented by the Johns Hopkins School of Medicine, evaluated the accuracy of Masimo SpHb—a noninvasive measurement of hemoglobin blood levels obtained using a Masimo Radical-7®Pulse CO-Oximeter™ with a Masimo rainbow®ReSposable Sensor. SpHb measurements obtained from 28 patients undergoing complex spine surgeries were compared with invasive blood samples drawn simultaneously and analyzed by a central laboratory. Results showed a mean bias of -0.3 for SpHb, a standard deviation of 1.0 g/dL and limits of agreement from -2.3 to 1.8 g/dL—demonstrating the "clinically acceptable accuracy" of SpHb when compared to a standard laboratory reference device. Researchers concluded that "continuous noninvasive SpHb monitoring may lead to earlier intervention and improved patient safety and care in the OR setting."1
According to lead researcher, Lauren Berkow, MD, Associate Professor of Anesthesiology and Critical Care Medicine at the Johns Hopkins School of Medicine, "The Masimo noninvasive hemoglobin monitor correlated strongly with gold-standard laboratory hemoglobin even during significant blood loss. The ability to continuously monitor hemoglobin during complex spine surgery may allow for earlier diagnosis and treatment of anemia related to surgical bleeding as well as prevent over-transfusion."
Traditional blood analysis has many drawbacks, including complexity, time-consuming turnaround times that can impact clinical decisions, and blood loss due to invasive blood draws that have been found to contribute substantially to the anemic conditions that commonly occur in critically ill patients—thereby increasing transfusion rates. Published studies have shown that transfusion of just one or two units of blood significantly increases infection, pneumonia, sepsis, and mortality after surgery.2-3 These studies also suggest that transfusions and their associated risks could be "largely avoided" through implementation of better blood management techniques and "more appropriate indicators" for transfusions.
The ability to noninvasively and continuously trend a patient's hemoglobin blood level during surgery allows for constant assessment of a patient's hemoglobin status, which may enable more appropriate clinical decisions—offering a breakthrough in blood management and patient safety that has the potential to improve patient outcomes, reduce patient exposure to unnecessary blood transfusions, and preserve precious blood resources.
According to Dr. Michael O'Reilly, Chief Medical Officer at Masimo, "Every surgical procedure bears certain risks—some may be preventable while others are unavoidable. Masimo SpHb fundamentally changes the way clinicians respond to bleeding and a patient's need for a blood transfusion by reducing measurement delays through real-time tracking and trending of hemoglobin blood levels. This study further underscores the value and impact of SpHb as a cost-effective solution to more accurately, easily, and rapidly monitor hemoglobin."
SpHb is available as part of the Masimo rainbow®SET platform—the first-and-only technology to noninvasively and continuously measure total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the 'gold-standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR).
1 Berkow L., Rotolo S., Mirski E. "Continuous and Noninvasive Hemoglobin Monitoring During Complex Spine Surgery." Johns Hopkins School of Medicine, Baltimore, Maryland. Presented at the STA Annual Meeting January 12-15, 2011.
2 Bernard AC et al. Intraoperative transfusion of 1U to 2U of packed red blood cells is associated with increased 30-day mortality, surgical site infection, pneumonia, and sepsis in general surgery patients. Journal of the American College of Surgeons. 2009;208:931-937.
3 Surgenor SD et al, for the Northern New England Cardiovascular Disease Study Group. The Association of Perioperative Red Blood Cell Transfusions and Decreased Long-Term Survival After Cardiac Surgery. Anesthesia & Analgesia 2009; 108:1741-1746.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, Pulse CO-Oximetry, Pulse CO-Oximeter, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57, Rad-8, Rad-5, Pronto-7, Pronto, Patient SafetyNet, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Happy Holidays from Masimo!
Irvine, California — December 21, 2011 — As we honor this very special time of the year and bid farewell to 2011 as a year of great challenge and growth in healthcare, it is with gratitude and a heart-felt desire to give something back to a few of the organizations that share our vision for better care and a better world, in your name. Simply respond with an email to charity@masimo.com specifying your selected charity choice from the list below and we will donate $10 in your name:
 |
We are grateful for the opportunity to affect positive changes that have transformed healthcare and we thank each of you for your support in helping us to make life better for the clinicians and patients we diligently serve.
Cheers to a new year full of great possibilities!
Masimo Mission Statement
Improving patient outcomes and reducing cost of care by taking noninvasive monitoring to new sites and applications.®
Masimo Guiding Principles
- Remain faithful to your promises and responsibilities.
- Thrive on fascination and accomplishment and not on greed and power.
- Make each day as fun as possible.
- Strive to make each year better than the year before, both personally and for the Team.
- Do what is best for patient care.
NOTE: Only e-mails sent to charity@masimo.com from official Livewire members will be processed. Please also include any comments or suggestions you might have that will help us to better fulfill our mission and adhere to our guiding principles.


Masimo to Present at 30th Annual J.P. Morgan Healthcare Conference
IRVINE, Calif., December 20, 2011 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the 30th Annual J.P. Morgan Healthcare Conference at the Westin St. Francis Hotel in San Francisco on Tuesday, January 10, 2012, at 3 p.m. Pacific Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOCT™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


New Clinical Study Presented at the NYPGA Meeting Shows Respiration Rate Monitoring— Considered the Fourth Primary Vital Sign—Has Been Revolutionized With Masimo rainbow Acoustic Monitoring Technology
Irvine, California – December 13, 2011 – Masimo (NASDAQ: MASI) announced today that a new clinical study evaluating Masimo Acoustic Respiration Rate (RRa™)was presented at the New York State Society of Anesthesiologists (NYSSA) Annual Post Graduate Assembly (PGA) Meeting in New York City.
The new study, presented at the 2011 NYPGA, highlights the positive clinical outcomes and patient safety impact of Masimo's unique Acoustic Respiration Rate (RRa™) measurement. Researchers Michael Ramsay, M.D., Elaine Lagow, R.N., and Mohammed Usman, Ph.D., at Baylor University Medical Center in Dallas, Texas, evaluated the accuracy and sensitivity of Masimo RRa and capnography and found that Masimo RRa had higher sensitivity for detecting respiratory pause events compared to capnography. The study, conducted in 34 post-surgical patients in the post-anesthesia care unit (PACU) each monitored for an average of 109 minutes, concluded that RRa provided "acceptable respiration rate accuracy" when compared to capnography, but only RRa had the additional advantage of providing "superior sensitivity for detecting respiratory-pause events" defined as no inspiration or expiration activity for > 30 seconds.1
Performance Characteristics of Masimo RRa and Capnography
(Compared to Reference Method) 1
| Method | Bias (bpm) | Standard deviation (bpm) | Sensitivity = TP/(TP+FN) | Specificity = TN/(TN+FP) |
|---|---|---|---|---|
|
Capnometry |
-0.6 |
2.6 |
62% |
98% |
|
RRa |
-0.1 |
2.4 |
81% |
99% |
Respiration rate is defined as the frequency of breathing expressed as the number of breaths per minute and is considered the fourth primary vital sign for assessing the physiological status of hospitalized patients; however, current methods for respiration rate monitoring are limited by reliability or patient tolerance. In contrast, Masimo rainbow Acoustic Monitoring technology is a breakthrough in respiration rate monitoring that is completely noninvasive, reliable, and virtually unnoticeable to the patient—enabling clinicians to continuously measure patient breathing acoustically to facilitate earlier detection of respiratory compromise and patient distress.
Featuring an innovative adhesive sensor with an integrated acoustic transducer that is applied to the patient's neck to detect upper airway acoustic vibrations on the surface of the skin during the respiratory cycle, Masimo rainbow Acoustic Monitoring uses patented acoustic signal processing that leverages Masimo's revolutionary Signal Extraction Technology (Masimo SET) to separate and process the respiratory signal and display continuous RRa measurements on the Radical-7 Pulse CO-Oximeter. RRa is part of Masimo rainbow®SET, a noninvasive patient monitoring platform enabling the measurement of multiple blood constituents, such as oxygen saturation, hemoglobin level, fluid responsiveness, respiration rate, and other physiological parameters that previously required invasive blood sampling and time-consuming laboratory analysis.
According to Dr. Ramsay, Chief of the Department of Anesthesiology and Pain Management at Baylor University Medical Center, "The increasing number of surgical patients with OSA and morbid obesity has increased our need for accurate surveillance. Our study findings showed that Masimo RRa was more accurate, sensitive, and specific to respiration rate changes than capnography. The improved accuracy offered by RRa translates to less false respiratory alarms, earlier detection of respiratory compromise and, ultimately, better patient care."
*To see a summary of all known clinical studies and abstracts on Masimo technologies and noninvasive measurements, please visit: https://professional.masimo.com/evidence/featured-studies/feature/.
1 Ramsay MA, Lagow E, Usman M. Accuracy of Respiration Rate and Detection of Respiratory Pause by Acoustic Respiratory Monitoring in the PACU. NYPGA 2011 Abstract #P-9137; presented December 11, 2011 @ 2 p.m.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®), PVI®, acoustic respiration rate (RRa™), and SEDLine®contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Antelope Valley Hospital Converts to Masimo rainbow SET for State-of-the-Art Noninvasive Patient Monitoring Capabilities
Hospital Standardizes to Pulse CO-Oximetry for Advanced Monitoring of Multiple Physiological Parameters without Invasive Procedures
Irvine, California - November 17, 2011 - Antelope Valley Hospital and Masimo (NASDAQ: MASI) today jointly announce the hospital's conversion to Masimo rainbow® SET Pulse CO-Oximetry™ technology-enabling advanced noninvasive patient monitoring capabilities that offer immediate, pain-free clinical data and physiological information. The hospital-wide conversion equips Antelope Valley's only full-service hospital with the most technologically and clinically-advanced oximetry and noninvasive patient monitoring solutions available-making patient monitoring both pain-less and cost-effective.
"Converting to Masimo rainbow® SET enables Antelope Valley Hospital to provide a higher level of monitoring care that is pain-free and less invasive than traditional capabilities," stated Edward Mirzabegian, CEO of Antelope Valley Hospital. "This new technology allows us to continuously track key blood, fluid, and respiration parameters without using invasive techniques to collect blood. It's like an invisible lifeline that provides our clinicians with immediate, real-time access to advanced clinical intelligence and continuous physiological measurements that were not available 10 years ago. And, because this technology is completely noninvasive, the cost-of-care is reduced to both the hospital and patient-enabling us to deliver an exceptional level of care and safety to our patients cost-effectively."
Masimo rainbow® SET Pulse CO-Oximetry is a breakthrough technology platform that allows hospitals to noninvasively measure and continuously monitor blood constituents and physiological parameters that previously required invasive procedures-including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the 'gold-standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR). The oximetry standard-of-care at leading hospitals worldwide, Masimo rainbow® SET provides immediate real-time results that enable clinicians to more rapidly assess patients and detect and treat adverse, potentially life-threatening conditions earlier.
Antelope Valley Hospital treats more than a quarter of a million inpatients and almost 100,000 emergency patients each year. This conversion ensures that all multiparameter patient monitors, stand-alone oximeters, and sensors feature the advanced noninvasive patient monitoring capabilities of Masimo rainbow® SET-allowing clinicians to easily and painlessly obtain and continuously track vital blood and physiological data for patients in real-time, anywhere in the hospital.
Laura Benesch, Assistant Chief Nursing Officer at Antelope Valley Hospital, said, "We see a lot of trauma patients where early detection and identification of distress is crucial to their care and survival. This is where our conversion to Masimo rainbow SET technology is truly beneficial because it offers us a noninvasive way of continuously assessing patients for key indicators of their physiological health status, like hemoglobin. When a patient is admitted to trauma/ED, OR, or ICU they will have a noninvasive sensor placed on their finger that will monitor their oxygen saturation and hemoglobin levels in real-time, along with seven other vital measurements to help clinicians detect abnormalities that may signal declining health status or a potentially life-threatening condition. This allows us to more tightly monitor our patients' health status to identify and treat problematic conditions earlier, before they become critical or life-threatening."
About Antelope Valley Hospital
Celebrating more than 50 years of caring for the community, Antelope Valley Hospital, a facility of Antelope Valley Healthcare District, is a non-profit, 420-bed hospital that was founded in 1955. The hospital is dedicated to providing quality care and services to everyone in the Antelope Valley. AV Hospital is located at 1600 West Avenue J in Lancaster, California. For further information, please visit www.avhospital.org or call 661-949-5000.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the hospital-wide conversion ensures that all Mater Children's Hospital patients will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo rainbow SET provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient; risks related to our belief that SpHb detects low or falling hemoglobin levels that could be the result of internal bleeding; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Julie Triggs
Antelope Valley Hospital
(661) 949-5530
julie.triggs@avhospital.org
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


Masimo to Present at 23rd Annual Piper Jaffray Health Care Conference
IRVINE, Calif., November 15, 2011 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the 23rd Annual Piper Jaffray Health Care Conference at The New York Palace in New York on Tuesday, November 29, 2011 at 9:30 a.m. Eastern Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


Shands Jacksonville Medical Center Converts to Masimo rainbow SET for State-of-the-Art Noninvasive Patient Monitoring Capabilities
Northeastern Florida Hospital with One of the Biggest and Busiest Trauma Centers Standardizes to Pulse CO-Oximetry for Advanced Monitoring of Multiple Physiological Parameters without Invasive Procedures
Irvine, California – November 10, 2011 – Shands Jacksonville Medical Center and Masimo (NASDAQ: MASI) today jointly announce the conversion of Shands Jacksonville Medical Center to Masimo rainbow®SET Pulse CO-Oximetry™ technology for advanced noninvasive patient monitoring capabilities. The conversion equips the acute care hospital, which also operates one of the biggest and busiest trauma centers, with the most technologically and clinically-advanced pulse oximetry technology—shown to have the best specificity (lowest rate of false alarms) and sensitivity (the highest true alarm detection)—and noninvasive patient monitoring solutions that allow clinicians to measure multiple blood constituents, fluid responsiveness, respiration rate, and other physiological parameters without invasive blood sampling and time-consuming laboratory analysis.1-2
"Converting to Masimo rainbow®SET provides our clinicians with superior patient monitoring capabilities and advanced clinical measurements that offer more immediate access to a higher quality of clinical data at the point-of-care," said Dr. Michael Nussbaum, Chair of the University of Florida College of Medicine Department of Surgery at Shands Jacksonville. "Our evaluations of available pulse oximetry technology led us to the decision to standardize on Masimo's technology platform not only because it works during motion and low perfusion, but because it also provides additional noninvasive blood, fluid and respiration capabilities that other oximeters can't—providing our clinicians with the clinical intelligence they need to deliver an exceptional level of care and safety to our patients."
Masimo rainbow®SET Pulse CO-Oximetry is a breakthrough technology platform that allows hospitals to noninvasively measure and continuously monitor blood constituents and physiological parameters that previously required invasive procedures—including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR). The pulse oximetry standard-of-care at leading hospitals worldwide, Masimo rainbow®SET provides immediate real-time results that enable clinicians to more rapidly assess patients and detect and treat adverse, potentially life-threatening conditions earlier.
Cynthia Gerdik, Director of Clinical Care Nursing at Shands Jacksonville, said the nursing staff relies on Masimo's ability to perform accurately under the most difficult of patient circumstances, "Masimo pulse ox technology is superb—even on patients with cold hands or our littlest pediatric 'wiggle worms', it just works. This was not the case with our prior pulse oximeter brand where it was not uncommon to get no or erroneous measurements on patients with cold extremities, poor perfusion, or even on patients who moved a lot. And, when you rely on pulse ox measurements as a vital sign for severely ill or trauma patients, accurate measurements are a must. Another indicator of Masimo's performance is the confidence our nurses have in its alarms. Today, when an alarm sounds on the Masimo oximeter, our nurses know that it's a real event that they need to immediately check. Masimo has freed us from alarm fatigue due to false alarms. We have trust in Masimo that we didn't have in our prior pulse ox solution."
Together, the University of Florida Health Science Center–Jacksonville and Shands Jacksonville form the region's premier academic health center–UF&Shands, a leader in the education of health professionals, a hub for clinical research and a unique provider of high-quality patient care in Northeast Florida. The conversion ensures that all multiparameter patient monitors, stand-alone oximeters, and sensors feature the advanced noninvasive patient monitoring capabilities of Masimo rainbow®SET—allowing clinicians to easily and painlessly obtain and continuously track vital blood and physiological data for patients in real-time, anywhere in the hospital.
1 Barker SJ. Motion-Resistant Pulse Oximetry: A Comparison of New and Old Models. Anesth & Analg. 2002 Oct;95(4):967-72.
2 Shah N, Estanol L. Comparison of Three New Generation Pulse Oximeters during Motion & Low Perfusion in Volunteers. Anesthesiology 2006;105:A929.
About Shands Jacksonville Medical Center
Shands Jacksonville is a private, not-for-profit hospital affiliated with the University of Florida Health Science Center campuses in Jacksonville and Gainesville. In Jacksonville, UF&Shands employs 5,000 physicians and caregivers and provides care to over 34,000 inpatients and 600,000 outpatients annually. To learn more about Shands Jacksonville, visit www.shands.org
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the hospital-wide conversion ensures that all Shands Jacksonville Hospital patients will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo rainbow SET provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient; risks related to our belief that SpHb detects low or falling hemoglobin levels that could be the result of internal bleeding; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dan Leveton
Shands Jacksonville
(904) 244-3268
daniel.leveton@jax.ufl.edu
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


Masimo Awarded the American Association of Respiratory Cares 2011 Zenith Award
Honored for the Fourth Straight Year at the International Respiratory Congress in Tampa, Florida
Irvine, California November 8, 2011 —Masimo (NASDAQ: MASI) announced today that it has received the American Association of Respiratory Care (AARC) prestigious Zenith Award for the fourth straight year. Honored at the 57th Annual International Respiratory Congress the largest gathering of respiratory care clinicians in the world—the AARC's Zenith Award represents Masimo's unwavering commitment, dedication, and industry leadership in providing best-in-breed, innovative patient care solutions.
AARC Executive Director and CEO, Sam Giordano, stated, "I congratulate Masimo for earning another Zenith Award this year. Companies receiving this prestigious award are chosen based on quality of products, truth in advertising, service, responsiveness, accessibility of sales staff, and support of the respiratory care profession. This year marks the fourth consecutive year that Masimo was voted to receive a Zenith by members of the American Association for Respiratory Care."
The leading national and international professional association for respiratory care, AARC encourages and promotes professional excellence, advances the science and practice of respiratory care, and serves as an advocate for patients and their families, the public, the profession and the respiratory care therapist. As the AARC's top industry honor for quality and service excellence, more than 400 companies were eligible for one of five Zenith Award honors. Award winners are chosen by the association's membership—more than 52,000 respiratory care professionals.
Joe Kiani, Chairman and CEO of Masimo, stated, "Respiratory professionals are a crucial component of the patient care team and their expertise is fundamental to patient recovery. We are proud of our long-standing commitment to the respiratory profession and are honored by the appreciation of so many AARC members who have once again recognized us with the Zenith Award for the superior quality and performance of our products and the service, commitment, and integrity excellence of our Team."
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient carehelping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb), oxygen content (SpOC™), carboxyhemoglobin (SpCO), methemoglobin (SpMet), and Pleth Variability Index (PVI), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa). Masimos rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications." Additional information about Masimo and its products may be found at www.masimo.com. *To see a summary of all known clinical studies and abstracts on Masimo technologies, please visit: https://professional.masimo.com/evidence/featured-studies/feature/.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


Masimo to Present at Lazard Capital Markets Healthcare Conference
IRVINE, Calif., November 8, 2011 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the Lazard Capital Markets Healthcare Conference at The Pierre Hotel in New York on Tuesday, November 15, 2011 at 1:00 p.m. Eastern Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


Masimo Reports Third Quarter 2011 Financial Results
Q3 2011 Highlights (compared to Q3 2010):
- Total revenue, including royalties, rose 3% to $104.0 million
- Product revenue rose 10% to $97.6 million
- 33,400 Masimo SET®and Masimo rainbow®SET units were shipped, increasing worldwide installed base by 16%
- Masimo rainbow revenue declined 35% to $7.8 million versus Q3 2010 when the company received an approximately $4 million order from the U.S. Marine Corps
- GAAP EPS was $0.24 versus $0.27 in the prior period, which included $0.01 in one-time expenses
Irvine, California, October 25, 2011 – Masimo (NASDAQ: MASI) today announced its financial results for the third quarter ended October 1, 2011.
Masimo's total third quarter revenue, including royalties, rose 3% to $104.0 million, compared to $101.0 million for 2010s third quarter. The company's third quarter product revenue rose 10% to $97.6 million, compared to $88.8 million for the third quarter of 2010. For the third quarter of 2011, the company's worldwide end-user business grew 18%, while OEM sales were down 20%. Revenue from Masimo rainbow products declined 35% to $7.8 million in the quarter, compared to $11.9 million for the third quarter of 2010 when Masimo received an approximately $4 million rainbow order from the U.S. Marine Corps.
Net income for the third quarter was $14.8 million, or $0.24 per diluted share, compared to reported net income of $16.4 million, or $0.27 per diluted share, in the third quarter of 2010, which included $0.01 per diluted share in one-time expenses.
During the third quarter, the company shipped approximately 33,400 Masimo SET pulse oximetry and Masimo rainbow SET Pulse CO-Oximetry units, excluding handheld units, down 11% compared to approximately 37,500 in the same prior year period. Masimo estimates its worldwide installed base as of October 1, 2011 to be 950,000 units, up 16% from 821,000 units as of October 2, 2010.
As of October 1, 2011, cash and cash equivalents were $143.2 million, compared to $88.3 million as of January 1, 2011.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "Masimo product revenue grew 10% in the period as strong SET performance was partially offset by declines in rainbow and OEM revenue. The 18% rise in our end-user, or direct, business was fueled by strong performance from our U.S. acute care business and even stronger performance from our international business. While we are disappointed with total rainbow revenue in the quarter, we remain confident in the power of rainbow parameters such as SpHb and RAM, which experienced strong year-over-year adhesive sensor unit and revenue growth in the quarter. We expect rainbow to not only help improve the quality and cost of patient care, but to be a catalyst for Masimo's revenue growth over the long term."
Revised Financial Guidance
Masimo now expects fiscal 2011 total revenue to be between $436 million and $439 million, including product revenue between $404 million and $407 million, and royalty revenue between $31.5 million and $32.5 million. Included within the revised 2011 product revenue range is a rainbow revenue expectation of $33 million to $35 million. The company now expects fiscal 2011 GAAP earnings per share to be between $1.04 and $1.06. Each of the components of Masimo's guidance set forth above is an estimate only and actual performance could differ.
Previously, the company's guidance was for fiscal 2011 total revenue to be between $446 million and $463 million, including product revenue at the lower end of the $415 million to $430 million range, and royalty revenue between $31 million and $33 million. Included within the previous product revenue range was a rainbow revenue expectation at the lower end of the $40 million to $50 million range. The company previously expected fiscal 2011 GAAP earnings per share to be at the lower end of the $1.17 and $1.25 range.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 18542610. After the live webcast, the call will be available on Masimo's website through November 25, 2011. In addition, a telephonic replay of the call will be available through November 8, 2011. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 18542610.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care...by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about: our financial condition, results of operations and business generally; our revised expectations for total revenue, royalty revenue and product revenue, including rainbow revenue, and GAAP earnings per share for the full fiscal year 2011; expectations regarding our ability to design and deliver innovative new noninvasive technologies; and global demand for our technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact: Sheree Aronson
Vice President, Investor Relations, Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact: Dana Banks
Manager, Public Relations, Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care...by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.





Guangdong Biolight Meditech Co., Ltd. Announces the Launch of Its Modular Patient Monitors with Masimo rainbow SET Pulse CO-Oximetry Technology
Irvine, California & Zhuhai, China – October 24, 2011 – Masimo (NASDAQ: MASI) and Biolight, a leading global manufacturer of electronic medical devices and patient monitors, today jointly announced both a worldwide technology licensing agreement and the launch of Masimo rainbow®SET Pulse CO-Oximetry technology in Biolight's new generation of modular patient monitors. The agreement allows Biolight to incorporate the breakthrough noninvasive blood constituent measurement capabilities of Masimo rainbow SET into Biolight's Anyview Series of modular monitors and the Measure-Through Motion and Low Perfusion pulse oximetry capabilities of Masimo SET across Biolight's product line of multiparameter patient monitors—facilitating early detection and treatment of life-threatening conditions.
According to James Yan, Chairman General Manager, Professor Senior Engineering, R&D. "Our corporate mission is to provide innovative medical devices with superior performance and value. The integration of Masimo rainbow SET Pulse CO-Oximetry and Masimo SET Measure-Through Motion and Low Perfusion pulse oximetry technologies is an example of our mission in action. Masimo's innovative technologies and breakthrough noninvasive monitoring solutions provide clinicians with the ability to more rapidly and thoroughly assess a patient's physiological status and make better clinical and treatment decisions."
Integrating Masimo rainbow SET Pulse CO-Oximetry technology into Biolight's Anyview Series of modular monitors will provide immediate and continuous access to additional clinical measurement data—enabling clinicians to detect and treat the early signs of hemodynamic instability, internal bleeding, and respiratory distress sooner. Masimo rainbow SET Pulse CO-Oximetry is a breakthrough technology platform revolutionizing patient monitoring by significantly expanding the ability to noninvasively measure and continuously track multiple blood constituents that previously required intermittent, invasive procedures, including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and perfusion index (PI), in addition to the 'gold standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), and pulse rate (PR).
"The inclusion of Masimo technologies will allow us to optimize the design and manufacture of state-of-the-art patient monitors and provide our domestic and international customers with access to the latest in cutting-edge, noninvasive physiological measurements," continued Yan. "As one of the first medical device manufacturers in China to incorporate Masimo's rainbow SET technology, we expect our combined product offering will allow clinicians to advance the delivery of healthcare across all patient care settings."
Incorporating Masimo's core Signal Extraction Technology (SET) pulse Oximetry technology into Biolight's non-modular "M" and "V" Series of patient monitors will also help clinicians more accurately measure a patient's oxygenation during challenging conditions such as patient motion and low perfusion to facilitate better treatment decisions – as clinically proven in over 100 independent studies. Utilizing patented signal processing technologies, including parallel engines and adaptive filters, Masimo SET delivers accurate and reliable measurements of a patient's true oxygenation status when conventional pulse oximetry technologies don't — reducing false alarms by over 95% (sensitivity) and expanding true alarm detection to over 97% (specificity).1
Rick Fishel, President of Worldwide OEM Business and Corporate Development at Masimo, stated, "Biolight is one of the first patient monitoring manufacturers in China to integrate Masimo rainbow SET Pulse CO-Oximetry technology. This "first-to-market" commitment in Biolight's modular Anyview series of patient monitors provides Biolight customers with advanced physiological monitoring across a broad range of monitors which offer a unique combination of upgradeability and point-of-care flexibility."
1 Shah N., Estanol L. "An Evaluation of Three New Generation Pulse Oximeters during Motion & Low Perfusion in Volunteers." Anesthesiology 2006; 102: S-75.
To see a summary of all known clinical studies and abstracts on Masimo technologies, please visit: https://professional.masimo.com/evidence/featured-studies/feature/.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
About Guangdong Biolight Meditech Co., Ltd.
Guangdong Biolight Meditech Co., Ltd. is a high-tech company located in Zhuhai, focusing on developing, producing and marketing medical instruments including patient monitoring systems, central monitoring systems, and electrocardiograph and digital colposcope systems. Founded in 1993, Biolight has successfully developed advanced medical electronic equipment in nearly 10 series and 40 types, including multi parameter patient monitors, central monitoring stations, and digital colposcope imaging systems. Biolight products have a considerable market share in China. We are also selling well in the international market and we have distributors in Asia, the Middle East, Europe and America. In 2003, Biolight built a new technology industrial park which covers 20,000sqm for developing and producing patient monitoring systems.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that the superior performance of Masimo SET pulse oximetry and the clinical importance of the upgradeable Masimo rainbow®SET Pulse CO-Oximetry™ technology platform have contributed to the growth and adoption of Masimo technologies, risks related to our belief that Masimo technologies help hospitals to improve patient care and enhance patient safety initiatives, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
Phone: (949) 297-7348
Email: dbanks@masimo.com
Thomas Lee
Biolight Co., Ltd.
Phone: 0086-756-3399996
Email: thomas@blt.com.cn
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.

New Clinical Studies Presented at the American Society of Anesthesiologists Annual Meeting Show Benefits of Masimo Noninvasive Technologies: SpHb, PVI, RRa, and SEDLine
Irvine, California – October 19, 2011 – Masimo (NASDAQ: MASI) announced today that over 25 new clinical studies evaluating Masimo noninvasive patient monitoring technologies were presented at the largest gathering of anesthesiologists in the world, the American Society of Anesthesiologists (ASA) Annual Meeting in Chicago, Illinois. The following studies highlight the positive clinical outcomes and patient safety impact of Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®), pleth variability index (PVI®), acoustic respiration rate (RRa™), and SEDLine®brain function monitoring.
SpHb®& PVI®
Researchers Steven Frank, M.D., James Rothschild, M.D., and John Ulatowski, M.D., at Johns Hopkins Hospital in Maryland found that the combination of continuous SpHb and PVI monitoring "may improve the efficacy and safety of the intraoperative autologous normovolemic hemodilution (IAHD) blood conservation technique that helps avoid allogeneic blood transfusions." The study, conducted in patients undergoing major abdominal or orthopedic surgery, concluded that SpHb was advantageous in "eliminating the delay in intraoperative decision-making that occurs when Hb measurements are obtained by conventional laboratory testing" while PVI was a "useful index of intravascular volume during the significant fluid shifts that occurred with IAHD, with increasing PVI indicating hypovolemia and decreasing PVI indicating adequate fluid resuscitation and blood re-infusion." 1
Two studies, conducted by the Long Beach Veterans Healthcare System in California, found that noninvasive SpHb measurements provided valuable clinical assessment information in remote, rural, and community settings. Nitin Shah, M.D., and Kinjal Shah, B.S., led a study using the Radical-7 in rural India, which concluded that SpHb measurements provided "reliable information compared to the invasive method" with a bias and precision of 0.2+/- 1.2 g/dL and was "very convenient to use in a rural camp setting" while also eliminating "biohazard risks of venipuncture and blood handling."2 The second study, led by Nitian Shah with Deval Modi, M.B, B.S., performed at two community health fairs in Long Beach concluded that the Pronto-7 spot-check device, with a bias and standard deviation of -0.06 +1.07 g/dL, provides "similar values and offers acceptable accuracy" when compared to values obtained from laboratory analysis of invasive blood samples. Researchers also noted the advantages of Pronto-7 in that it "gives immediate result" and has the potential to be "very helpful in spot checking for anemia in the general community without the need for exhaustive set-up or processing time."3
Two separate studies showed that the new In Vivo Adjustment™ feature for noninvasive SpHb measurements, included as part of the new 2011 Radical-7, along with the newest generation rainbow ReSposable Sensors (Rev. E) helped clinicians to improve the agreement in subsequent comparisons between invasive (tHb) and noninvasive (SpHb) hemoglobin measurements. Researchers Kathleen Richard, M.D., Timothy Quill, M.D., Thomas Dodds, M.D., and Matthew Koff, M.D., M.S., at Dartmouth Hitchcock Medical Center in New Hampshire found that the "accuracy and descriptive statistics improved when each time-matched SpHb value was adjusted by the magnitude of difference between the first tHb and corresponding SpHb." Researchers concluded that "if this type of adjustment were performed in real-time in vivo (intra-operatively), overall accuracy of SpHb values may be further improved," which may also "add value to care" during high risk surgical procedures in patients with co-morbidities and "in many other clinical locations and scenarios (ICU, PACU, pediatrics, emergency medicine, surgical ward)."4 Another study, conducted by Ryo Miyashita, M.D., Shigekazu Sugino, M.D., Yukitoshi Niiyama, M.D., Mitsuko Mimura, M.D., Ph.D., and Michiakii Yamakage, M.D., Ph.D., in Japan at the Sapporo Medical University School of Medicine, found that "SpHb values corrected using the novel program were more accurate than those obtained conventionally." Study results showed that In Vivo Adjustment improved bias and standard deviation from 1.1 + 1.0 to 0.0 + 0.61 g/dL) and concluded that In Vivo Adjustment should "contribute to the accurate measurement of SpHb in the clinical setting."5
Researchers Peter Winch, M.D., Aymen Naguib, M.D., Julie Rice, R.N., and Joseph Tobias, M.D., at Nationwide Children's Hospital showed that SpHb measurements obtained in pediatric patients undergoing acute blood loss correlated to hemoglobin values obtained from a point-of-care device. Researchers evaluated the use of SpHb in a pediatric population undergoing phlebotomy and concluded that SpHb provided "accurate and continual real-time data which can inform medical care".6
In a separate study, also conducted at Nationwide Children's Hospital, the same research team found that monitoring changes in PVI could be used as a guide for volume replacement during isovolemic hemodilution in pediatric patients undergoing congenital cardiac surgery. Patients were evaluated in two groups—group 1 had starting PVI values less than 14 and group 2 had starting PVI values greater than 14. Results showed that the average crystalloid replacement in group 1 was 5ml/kg, while volume replacement in group 2 was 11ml/kg in order to maintain the same hemodynamics during hemodilution. Researchers noted that the "data demonstrates the possible advantage of using the PVI value as a tool for identifying patients who would be good candidates for isovolemic hemodilution."7
Rainbow Acoustic Monitoring™ for RRa™
Researchers Basavana Goudra, M.D., and Lakshmi Penugonda, M.D., at the Hospital of the University of Pennsylvania, evaluated RRa measurements obtained using the Masimo Rad-87 during upper gastrointestinal endoscopy and found it had the "best accuracy and precision (-0.3 +/- 1.0 bpm) of monitoring respiration rate," whereas EtCO2 (-0.6 +/- 6.1 bpm) and impedance pneumography (0.2 +/- 4.3 bpm) are "subject to frequent false alarms." Results showed that EtCO2 had the "highest incidence of false alarms" with 45 false alarms out of 52 events, while RRa had the "lowest rate of false alarms" with just 3 out of 52 events.8
SEDLine®
In a study conducted at Loma Linda University Medical Center, researchers Kevin Nasseri-Noori, M.D., Deborah McIvor, M.D., Frank Hsu, M.D., Moses Olson, B.S., Martin Allard, M.D., Travis Losey, M.D., Mark Macknet, M.D., and Richard Applegate, M.D., demonstrated that the SEDLine 4-channel (PSA array) brain function monitor "correctly detected an excessively deep intraoperative burst suppression therapy (IBST) pattern given a nearly isoelectric EEG" in 16 out of 19 events during intracranial surgery. With an overall correlation of 93% between SEDLine (PSA array) and EEG—showing strong agreement—study results indicate that SEDLine may be an effective tool for IBST detection versus standard EEG, which is expensive and requires a special technician in the room.9
According to Nitin Shah, MD, Chief of Surgical ICU at Long Beach VA Hospital and Professor of Anesthesiology at Loma Linda University, "Noninvasive SpHb is the way of the future. It is truly a blessing for a developing country with limited resources. Patients appreciate that it eliminates the need for traditional needle stick blood draws, so they are much more relaxed and willing to complete the pain-free SpHb testing. And, for healthcare professionals it is unbelievable. There are no biohazard risks, no formal training required to operate, and no calibration required with SpHb testing and they get instant results that they can use immediately to advise their patients right on the spot. In my view, SpHb should be routinely used in clinics and community settings across the globe."
Basavana Goudra, MD, Assistant Professor of Anesthesiology and Critical Care at the Hospital of the University of Pennsylvania, commented, "Masimo rainbow acoustic monitoring is better than anything else we have at the moment for accurate and reliable respiration rate measurements. Not only was it easy to use in the majority of the patients enrolled in our study, but our results show that it is the perfect solution for routine use in upper GI endoscopy procedures and post-operative monitoring as EtCO2 is not useful in these situations.
1 Frank S, Rothschild J, Ulatowski J. "Continuous Noninvasive Hemoglobin Monitoring for Jehovah's Witness Patients Undergoing Intraoperative Autologous Normovolemic Hemodilution" ASA 2011 Presentation A408.
2 Shah N, Shah K. "Evaluation of a Pulse CO-Oximeter for Noninvasive Hemoglobin Measurement in Adult Population in Rural India" ASA 2011 Presentation A283.
3 Shah N, Modi D. "Accuracy of Noninvasive Hemoglobin Measurement Through a Pulse CO-Oximeter Compared to Venous Blood Draw in a Community Setting" ASA 2011 Presentation A285.
4 Richard K, Quill T, Dodds T, Koff M. "Improved Accuracy and Trending of Noninvasive Hemoglobin Measurements with 'In Vivo Adjustment'" ASA 2011 Presentation A1667.
5 Miyashita R, Sugino S, Niiyama Y, Mimura M, Yamakage M. "Improved Noninvasive Total Hemoglobin Measurements After In Vivo Adjustment" ASA 2011 Presentation A410.
6 Winch P, Naguib A, Rice J, Tobias J. "The Accuracy of Noninvasive Hemoglobin Monitoring Following Phlebotomy in a Pediatric Patient Population" ASA 2011 Presentation A411.
7 Naguib A, Winch P, Rice J, Tobias J. "Can the Starting Pulse Oximeter Derived Pleth Variability Index (PVI) Predict Total Crystalloid Replacement During Isovolemic Hemodilution in Congenital Cardiac Surgery?" ASA 2011 Presentation A207.
8 Goudra B., Penugonda L. "Monitoring Respiration in Upper GI Endoscopy Anesthesia" ASA 2011 Presentation A246.
9 Nasseri-Noori K, McIvor D, Hsu F, Olson M, Allard M, Losey T, Macknet M, Applegate R. "Prospective Comparison of Global Electroencephalogram to Frontal SEDLine Electroencephalogram Monitoring for the Evaluation of Intraoperative Burst Suppression During Elective Intracranial Surgery" ASA 2011 Presentation A499.
*In-Vivo Adjustment is pending FDA 510(k) clearance.
**To see a summary of all known clinical studies and abstracts on Masimo technologies and noninvasive measurements, please visit: https://professional.masimo.com/evidence/featured-studies/feature/.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com .
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®), PVI®, acoustic respiration rate (RRa™), and SEDLine®contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo to Report Third Quarter 2011 Financial Results after Market Close on October 25, 2011
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., October 13, 2011 -- Masimo (NASDAQ: MASI) announced today that it will release third quarter financial results for the period ended October 1, 2011, after the market closes on October 25, 2011. The conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 18542610. After the live webcast, the call will be available on Masimo's website through November 25, 2011. In addition, a telephonic replay of the call will be available through November 8, 2011. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 18542610.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact: Media Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Launches the 2011 Radical-7 with rainbow® Acoustic Monitoring and In Vivo Adjustment™ 2011 Radical-7 will be Shown October 15-19 at the 2011 American Society of Anesthesiologists (ASA) Annual Meeting in Chicago
Irvine, California – October 13, 2011 – Masimo (NASDAQ: MASI) announced today FDA 510(k) clearance and CE Mark of its new Radical-7®with rainbow®Acoustic Monitoring capability for continuous display of the acoustic respiration rate (RRa™) waveform and measurements. Also new to the Radical-7 is an In Vivo Adjustment™ (pending FDA 510(k) clearance in the U.S.) that allows clinicians to, for the first time, adjust the noninvasive measurements to the specific patient and laboratory reference device they use for invasive blood testing.
rainbow®Acoustic Monitoring
The new Radical-7 provides clinicians with the option to measure RRa and display the acoustic waveform directly on the monitor screen with the pleth waveform overlayed—allowing them to observe changes in breathing upon inhalation and exhalation. With this new display capability, clinicians may be able to more readily detect respiratory pause events where there is an absence of breathing, high ambient noise that can degrade the acoustic signal, and improper sensor placement.
 |
Respiration rate is defined as the frequency of breathing expressed as the number of breaths per minute and is considered a critical vital sign in assessing the physiological status of hospitalized patients; however, current methods for respiration rate monitoring are limited by reliability or patient tolerance. In contrast, Masimo rainbow Acoustic Monitoring is completely noninvasive and virtually unnoticeable to the patient—featuring an innovative adhesive sensor with an integrated acoustic transducer that is easily and comfortably applied to the patient's neck to detect upper airway acoustic vibrations on the surface of the skin during the respiratory cycle. Using patented acoustic signal processing that leverages Masimo's revolutionary Signal Extraction Technology (SET), the respiratory signal is separated and processed to display continuous RRa measurements.
In Vivo Adjustment Allows Users to Account for Individual Patient Bias from Laboratory Reference Device
The measurement of blood components such as oxygen saturation, methemoglobin, carboxyhemoglobin, and total hemoglobin have inherent and expected variability within and between noninvasive and invasive measurement techniques and from patient to patient. For example, all of the noninvasive measurements use a technique coined 'patient calibration' which takes the noninvasive monitor's detected light that is transmitted or reflected from the patient at different values and matches them to empirically gathered invasive blood measurements from a small number of volunteers and in some cases patients. However, individuals may vary from the pool of patients used in the 'patient calibration' and hence provide a bias for the particular patient. In addition, many clinicians are unaware that laboratory reference devices that measure oxygen saturation or hemoglobin can have great variability from each other. In fact, hemoglobin can vary 2 g/dL or more from one manufacturer's product to another. And, physiologic factors such as the blood source (venous or arterial), site and time of blood draws, blood draw technique, and patient body position are recognized in the clinical literature to add variability to hemoglobin levels. As a result, noninvasive and invasive measurements can vary in the same patient depending on the methods and invasive equipment used.
The In Vivo Adjustment feature available in the new Radical-7 now provides the option to adjust the noninvasive oxygen saturation (SpO2), total hemoglobin (SpHb®), carboxyhemoglobin (SpCO®), and methemoglobin (SpMet®) values displayed on the monitor to the laboratory reference value used at the site by patient. With this new feature, clinicians can adjust the noninvasive value at the beginning of a monitoring period to account for individual patient variation and the laboratory reference value and continuously trend the noninvasive value to the patient and reference laboratory value until the next blood sample and laboratory analysis.
"With the addition of the new In Vivo Adjustment feature, Masimo refines its solution for three major limitations that have always plagued pulse oximetry: 1) the inability to measure-through motion and low perfusion (virtually eliminated by Masimo SET); 2) the inaccuracy of SpO2 in the presence of dyshemoglobins (they can now be monitored with SpCO and SpMet); and 3) the inability of one calibration curve to fit all patients (an individual patient's SpO2 can be adjusted to a reference arterial blood gas measurement)," stated Steven Barker, PhD, MD, Chairman of Masimo's Scientific Advisory Board.
Juan Soliveres, M.D., University Hospital in Valencia, Spain, stated, "There are many invasive devices that measure Hb and the results they report may be different from one device to the next. Even laboratory co-oximeters will report different Hb values when the same blood sample is run through different machines of the same make and model. When comparing invasive Hb measurements with noninvasive SpHb measurements, an In Vivo Adjustment feature—to remove measurement bias and calibrate SpHb measurement relative to the lab device—is an important enhancement that should contribute to improved Hb correlations between these measurements."
In addition, the new Radical-7 now features a larger number display with pulse oximetry measurements that are 40% larger than before to improve visibility. And for added safety, the device now includes an optional Alarms All Mute feature that allows the customer to enable or disable the ability for users to mute (silence) alarms on the monitor and adds a layer of protection that password-protects this feature—enabling only qualified clinicians to turn-on the Alarms All Mute feature. This feature safeguards against indiscriminate and accidental silencing of monitor alarms that can place patients at increased risk of unrecognized distress and deterioration, while enabling qualified clinicians (through password access) the ability to focus their attention on immediate patient care, interventions, and intensive care resuscitations in sensitive care situations without the audible distractions of monitor alarms.
Masimo Founder and CEO, Joe Kiani, stated, "With the latest Radical-7, we have once again expanded what clinicians can expect from a pulse oximeter; this time by adding the ability to monitor comfortably and reliably a patient's respiration rate. And, with In-Vivo Adjustment, clinicians can, when they need to, get very accurate measurements that are not only patient focused, but adjusted to their laboratory reference devices."
According to Michael O'Reilly, MD, Chief Medical Officer at Masimo, "On hospital general care floors around the world, the problems associated with aggressive post-operative pain management with patient-controlled analgesia (PCA) pumps, rising patient co-morbidities, and decreasing nurse-to-patient ratios have led to dramatic increases in adverse events and avoidable patients deaths. As a result, leading patient safety organizations have been calling for continuous monitoring of oxygenation and ventilation on post-surgical general care floor patients. Today, thanks to Masimo innovations, the need for a reliable SpO2 and respiration rate monitor for patients on general care floors is finally realized—and patients can be safer than ever before."
*The new Radical-7 Pulse CO-Oximeter has FDA 510(k) and CE Mark clearances for rainbow®Acoustic Monitoring and CE Mark clearance for In-Vivo Adjustment. In-Vivo Adjustment is pending FDA 510(k) clearance.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com .
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that the new Radical-7 offers clinicians expanded functionality and new features that improve patient monitoring; risks related to our belief that the new RRa waveform overlayed with the pleth waveform will allow clinicians to more readily detect respiratory pause events where there is an absence of breathing, high ambient noise that can degrade the acoustic signal, and improper sensor placement; and risks related to our belief that the new In Vivo Adjustment feature will enable clinicians to calibrate noninvasive measurements directly to their specific invasive blood sampling reference device for improved device-to-device comparisons; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo Rainbow Acoustic Monitoring Technology Cleared for Market in Japan Acoustic Respiration Rate (RRa) Technology Now Available for First Time in Japan in the Masimo Rad-87 Pulse CO-Oximeter
Tokyo, Japan and Irvine, California – October 10, 2011 – Masimo (NASDAQ: MASI) announced today that it has received Japanese Ministry of Health Labor & Welfare (MHLW) regulatory clearance for its Rad-87®Pulse CO-Oximeter with rainbow®Acoustic Monitoring technology—providing noninvasive and continuous acoustic respiration rate (RRa™) measurements that are accurate, easy-to-use, and enhance patient compliance. Available for the first time in Japan, RRa represents a breakthrough solution for measuring respiration rate in real-time to track and trend patient breathing—offering automated assessments that may enable earlier detection of respiratory compromise and patient distress.
Respiration rate is defined as the frequency of breathing expressed as the number of breaths per minute and is considered a critical vital sign in assessing the physiological status of hospitalized patients. Continuous monitoring of respiration rate is especially important for post-surgical patients receiving patient-controlled analgesia (PCA) for pain management as sedation can induce respiratory depression and place patients at considerable risk of serious injury or death.1-4 Although the Anesthesia Patient Safety Foundation (APSF) recommends continuous oxygenation and ventilation monitoring in all patients receiving opioids,5-6 current methods for respiration rate monitoring are limited by reliability or patient tolerance.
Dr. Toshiyasu Suzuki, MD, PhD, Professor of Anesthesiology, Tokai University School of Medicine in Kanagawa Japan, who is one of the first Rad-87 with RRa users, stated, "The number of surgeries has been increasing at acute care hospitals since DPC started in Japan. As a result, the number of postoperative patients sent to the general ward has increased and nurses have more workload, especially at the night shift. We have concerns about patient safety. Anesthesiologists tend to administer postoperative patients muscle relaxant and/or pain relief drugs such as Morphine, Fentanyl, Remifentanil. Those patients are often at risk of respiratory failure when excessive amounts of these drugs are administered. The respiratory monitoring for those patients is currently done by pulse oximeter and/or a visual observation, however that is not enough. Now we have a solution. I strongly recommend Rad-87 with RRa: it is a simple use and the RRa accuracy is as good as ETCO2, on top of that SpO2 can be monitored at the same time. I am assured that Rad 87 with RRa will be a powerful tool for clinicians and nurses on a postoperative patient risk management."
Michael Ramsay, M.D., Chief of the Department of Anesthesiology and Pain Management at Baylor University Medical Center in Dallas, Texas, stated, "Breathing adequately is what matters most. Masimo acoustic respiration rate (RRa) provides clinicians with the ability to automatically and continuously monitor the breathing status of post-surgical patients in general care or post-anesthesia settings—alerting them to the first sign of an abnormal or compromised breathing pattern that may be indicative of airway obstruction or respiratory distress. And, the Masimo Acoustic Sensor is virtually unnoticeable when worn—making it extremely patient-friendly."
Masimo rainbow Acoustic Monitoring features an innovative adhesive sensor with an integrated acoustic transducer that is easily and comfortably applied to the patient's neck to detect upper airway acoustic vibrations on the surface of the skin during the respiratory cycle. Using patented acoustic signal processing that leverages Masimo's revolutionary Signal Extraction Technology (SET), the respiratory signal is separated and processed to display continuous RRa measurements.
Masimo Founder and CEO, Joe Kiani, stated, "Solving 'unsolvable' problems that have plagued patient monitoring is key to advancing medicine and improving patient care. Masimo Rainbow Acoustic Monitoring addresses a longstanding and growing need to accurately and easily monitor patient breathing, which is expected to improve patient safety and decrease the cost of care in hospitals. It also adds another compelling reason for hospitals to choose the Masimo rainbow SET upgradeable platform for pulse oximetry, Pulse CO-Oximetry, and now Acoustic Monitoring."
1 Joint Commission on Accreditation of Healthcare Organizations. Sentinel Event Alert: Patient controlled analgesia by proxy. Chicago: JCAHO, 2004
2 Institute for Safe Medication Practice. Safety issues with patient-controlled analgesia: Part 1-How errors occur. Huntingdon Valley: ISMP, 2003
3 Institute for Safe Medication Practices. Safety issues with patient-controlled analgesia: Part II - How to prevent errors Huntingdon: ISMP, 2003
4 Bird M. Acute pain management: a new area of liability for anesthesiologists ASA Newsletter. Park Ridge: American Society of Anesthesiologists, 2007
5 Weinger MB. Dangers of Postoperative Opioids: APSF Workshop and White paper address prevention of postoperative respiratory complications. APSF Newsletter, 2006;21:61-7. http://www.apsf.org/newsletters/html/2007/winter/winter2006-7.htm
6 Stoelting RK, Weinger MB. Dangers of Postoperative Opioids -- Is There a Cure? APSF Newsletter, 2009;24:25-26. http://www.apsf.org/newsletters/html/2009/summer/index.htm
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo rainbow acoustic monitoring technology and RRa respiration rate measurements provide accurate, reliable, real-time data for all patients under all conditions and offer automated assessments, which may enable earlier detection of respiratory compromise and patient distress, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium


Masimo Achieves New Milestone-Ships One Millionth Oximeter
Milestone Event Celebrates Wellness and Commitment to Advancing the Standard-of-Care
Irvine, California - September 29, 2011 - Masimo (NASDAQ: MASI) announced today that it shipped its 1,000,000th Masimo SET®pulse oximeter and rainbow®SET Pulse CO-Oximeter (excluding hand-held units). The one millionth oximeter went to Sisters of Charity Hospital in Buffalo, New York, as part of a new NICU, Labor & Delivery and Nursery technology conversion. To celebrate this milestone, Masimo held a Millionth Oximeter Celebration at its corporate headquarters in Irvine, California today.
Joe Kiani, Founder, chairman and CEO of Masimo, stated "We are excited to achieve this milestone and share the moment with the Sisters of Charity Hospital and our local community. As the recipient of our millionth Masimo rainbow SET oximeter, Sisters of Charity Hospital joins an ever-growing list of premier hospitals that have chosen Masimo
to provide the new standard-of-care in patient monitoring. And, celebrating our millionth oximeter is a reminder of how our technologies have advanced patient care and improved patient safety for millions."
Founded with the goal of solving the 'unsolvable' problems plaguing patient care, Masimo innovations have revolutionized pulse oximetry and noninvasive blood constituent, hemodynamics, and respiration monitoring. When conventional pulse oximetry technologies failed to measure accurately and reliably during patient motion and low perfusion and other companies could not solve the problem, Masimo succeeded with the invention of Masimo SET®Measure-Through Motion and Low Perfusion pulse oximetry technology. When conventional pulse oximetry failed to measure accurately in the presence of dyshemoglobins (like carboxyhemoglobin and methemoglobin) and other pulse oximetry technologies could not isolate and measure dyshemoglobins, Masimo succeeded again. With Masimo rainbow®SET Pulse CO-Oximetry™ clinicians can now continuously measure and monitor multiple blood constituents, dyshemoglobins, and physiological parameters that previously required invasive procedures. And, thanks to Masimo Patient SafetyNet™, continuous patient monitoring on general care floors-where 80% of the hospital population is cared for-is now finally a reality at hospitals worldwide.
"When we started Masimo, our mission was to 'improve patient outcome and reduce cost of care by taking noninvasive monitoring to new sites and applications.' While we feel like we have just begun, I am proud that we have already accomplished every aspect of our mission," continued Joe Kiani.
Today, Masimo medical breakthroughs and technology innovations allow more patients to be accurately and reliably monitored and are transforming lives in ways that were not possible with conventional pulse oximetry. Spanning every care area and clinical setting, Masimo solutions are helping clinicians to improve patient safety and clinical outcomes by dramatically increasing the early detection of physiological abnormalities and life-threatening conditions, reducing retinopathy of prematurity (ROP), enabling the screening of congenital heart defects (CHD) in newborns, and decreasing risky blood transfusions in the operating room, as well as rescue events and costly ICU transfers on general care floors are just some of the ways Masimo is making a measureable difference for patients around the world.
According to Antonio Garcia at Frost & Sullivan, "Masimo simply takes the guess work out of pulse oximetry, so clinicians never have to guess what brand is the most reliable. Masimo's novel product portfolio has added a new dimension to noninvasive patient monitoring equipment that has considerably facilitated early detection of challenging patient conditions."
The superior performance and innovative capabilities of Masimo SET and rainbow SET technologies and products have produced more than 12 medical firsts, garnered over 60 industry awards, and fueled the growing adoption of Masimo at hospitals around the world. In just four years, Masimo has doubled its 2007 milestone of 500,000 oximeters shipped on the heels of its 200,000th oximeter milestone, achieved in 2004. And, today more than half of the top hospitals listed on the U.S. News & World Report Best Hospitals Honor Roll have converted to Masimo technology.
Masimo SET has been clinically proven in over 100 independent studies to provide the most accurate and reliable SpO2 and pulse rate measurements, even under the most challenging conditions of patient motion and low peripheral perfusion. Utilizing patented signal processing technologies, including parallel engines and adaptive filters, Masimo SET delivers accurate and reliable measurements of a patient's true oxygenation status when conventional pulse oximetry technologies don't reducing false patient monitor alarms by over 95% (sensitivity) and expanding true alarm detection to over 97% (specificity).1 Before Masimo SET, pulse oximetry was a fair weather friend-useful only under ideal conditions, but unreliable when accurate monitoring was needed most. With Masimo SET, pulse oximetry is now not only a foul weather friend, but is being used in places and care areas it couldn't before. As a result, the industry has not only come to depend on continuous noninvasive physiologic monitoring by Masimo SET pulse oximetry, but has made it the leading Measure-Through Motion and Low Perfusion SpO2 solution incorporated into over 100 multiparameter monitors and 50 monitoring brands.
In addition, Masimo has taken noninvasive monitoring to new applications by bringing new noninvasive capabilities and solutions to the point-of-care that eliminate the pain, complexity, and delays associated with invasive blood draws and procedures. Masimo rainbow SET®Pulse CO-Oximetry™, a breakthrough technology platform featuring the accuracy and reliability of Masimo SET, is continuing to revolutionize patient monitoring by significantly expanding the ability to noninvasively capture, continuously track and monitor multiple blood constituents that previously required invasive procedures, including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), perfusion index (PI), and acoustic respiration rate (RRa™), in addition to the 'gold standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), and pulse rate (PR).
Over the Years, Leading Clinicians Have Recognized Masimo Innovations as Medical Breakthroughs
In 2002, Dr. Charles Durbin, Department of Anesthesiology, at the University of Virginia Health System in Charlottesville, Virginia, warned clinicians of the impact of conventional pulse oximetry on patient care and safety, stating that, "Monitors providing false alarms distract caregivers from other tasks and require attention to trouble shoot or fix the monitor. This decreases efficiency, increases costs, and increases the likelihood that monitoring will not be continued because of caregiver distrust of the device. Ultimately, patient safety may be adversely affected. This is particularly important because oximetry is being used outside the ICU on the general ward where the effects of false alarms are even more likely to negatively affect patient outcome. Recent studies on human error and patient safety point to caregiver cognitive overload and distraction (termed latent conditions) as one cause of patient injury or error." In contrast to conventional oximetry, Dr. Durbin's research studies concluded that "Masimo SET pulse oximetry technology was more reliable and accurate than conventional pulse oximetry, has been shown to perform well in poor perfusion states and during motion, resulted in far less monitoring failure time than conventional oximetry, and presented more reliable oximetry data to clinicians, which resulted in 68% faster weaning of FI02 with 34% fewer arterial blood gas draws (ABGs)."2
In 2003, Dr. Augusta Sola, lead researcher and author of a breakthrough protocol to reduce ROP using Masimo SET pulse oximetry stated, "I tell my friends, my family, and my neighbors in America and overseas, a good thing happened in 1989-Masimo SET technology changed the way we can use pulse oximetry." Studies conducted at Cedars-Sinai Medical Center in Los Angeles showed that Masimo SET helped to reduce the rate of ROP from 12% to 2.5% and the need for ROP laser treatment from 4.5% to 0%.3
In 2005, Dr. Steven Barker, Head of the Department of Anesthesiology at the University of Arizona College of Medicine, commented that, "In the 25-year history of pulse oximetry, no one has produced an instrument that will function accurately on patients suffering from carbon monoxide poisoning. The development of a pulse oximeter that can separately measure carboxyhemoglobin and oxyhemoglobin will be a major advance in healthcare…and would soon become a standard of care in emergency rooms, ambulances, and many other critical care settings."4
Numerous lifesaving accounts have highlighted the impact of noninvasive carboxyhemoglobin (SpCO) measurements from Masimo's handheld Rad-57, including one from Wake County EMS Chief, Skip Kirkwood, M.S., J.D., EMT-P, who said, "We believe that all 50+ hotel guests might have been dead at dawn if it were not for this lifesaving intervention from Masimo."
Masimo's First Customers are Still Ardent Supporters and Users of the Technology Almost 20 Years Later
One of Masimo's first installations was at Hannover Medical School in Hannover, Germany, where Prof. Christian F. Poets, M.D. was the Director of the Neonatal Intensive Care Unit (NICU) at the time. Now the Medical Director for the Department of Neonatology at Tubingen University Hospital (Germany), Dr. Poets stated, "In my view, Masimo has revolutionized the continuous, noninvasive measurement of oxygenation by making it much more motion-resistant and capable of producing reliable measurements at low perfusion. This has been made possible by excellent engineering and their willingness to listen to their customers' needs."
Dr. Katsuyuki Miyasaka, Director of the Perioperative Center at St. Luke's International Hospital in Tokyo, Japan, is another one of Masimo's very first customers, responsible for the first hospital-wide installation of Masimo SET pulse oximeters at the National Center for Child Health and Development (Tokyo). "When I first experienced using a Masimo SET pulse oximeter on a hyperkinetic infant in 1993, I was amazed and assured that Masimo SET would be the first-ever pulse oximeter to solve the problem of patient motion artifact and low perfusion. Congratulations to Masimo for shipping one million pulse oximeters. I am sure that SpHb and other emerging noninvasive innovations enabled by Masimo rainbow SET technology will further contribute to improved patient care and save even more patients' lives."
Peter U. Bergmann, FACHE, President and CEO of Sisters of Charity Hospital and one of Masimo's newest customers, stated, "We are honored to be the recipient hospital of Masimo's millionth oximeter. Converting the NICU, labor & delivery and nursery departments to Masimo SET provides us with the most advanced oximetry technology enabling the most advanced care for newborns. At Sisters of Charity Hospital, you can be confident that your baby is receiving the most advanced medical and nursing care available using state-of-the-art technology with a family-centered approach to care. Our number one priority is to ensure that you and your baby receive the best care possible during your stay with us and Masimo oximeters are an important part of monitoring our tiniest patients."
1 Shah N., Estanol L. "An Evaluation of Three New Generation Pulse Oximeters during Motion & Low Perfusion in Volunteers." Anesthesiology 2006; 102: S-75.
2 Durbin C.G. Jr., Rostow S.K. "More Reliable Oximetry Reduces the Frequency of Arterial Blood Gas Analyses and Hastens Oxygen Weaning after Cardiac Surgery: A Prospective, Randomized Trial of the Clinical Impact of a New Technology." Crit Care Med. 2002 Aug;30(8):1735-40.
3 Chow L.C., Wright K.W., Sola A.; CSMC Oxygen Administration Study Group. "Can Changes in Clinical Practice Decrease the Incidence of Severe Retinopathy of Prematurity in Very Low Birth Weight Infants?" Pediatrics. 2003 Feb;111(2):339-45.
4 Barker S.J., Curry J., Redford D., Morgan S. "Measurement of Carboxyhemoglobin and Methemoglobin by Pulse CO-Oximetry: A Human Volunteer Study." Anesthesiology. 2006 Nov;105(5):892-7.
To see a summary of all known clinical studies and abstracts on Masimo technologies, please visit: https://professional.masimo.com/evidence/featured-studies/feature/.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
About Sisters of Charity Hospital
Sisters of Charity Hospital was Buffalo's first hospital, with a tradition of superior care dating back to 1848. Today, the hospital is part of the Catholic Health system that provides care to Western New Yorkers across a network of hospitals, primary care centers, imaging centers, and several other community ministries. With 290 licensed beds, Sisters of Charity Hospital provides comprehensive medical care and treatment to more than 15,000 inpatients, 33,000 emergency room visitors, and 560,000 outpatients each year.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that the superior performance of Masimo SET pulse oximetry and the clinical importance of the upgradeable Masimo rainbow®SET Pulse CO-Oximetry™ technology platform have contributed to the growth and adoption of Masimo technologies, risks related to our belief that Masimo technologies help hospitals to improve patient care and enhance patient safety initiatives , as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Published Study Demonstrates the Absolute and Trend Accuracy of Masimo Noninvasive and Continuous Total Hemoglobin (SpHb) Monitoring inIntensive Care Unit Patients
Researchers Cite Masimo SpHb as a "Significant Expansion of Existing Monitoring Technology"
Irvine, California - September 27, 2011 -Masimo (NASDAQ: MASI) announced today that findings from a new study published in the Journal of Critical Care Medicine demonstrate that noninvasive and continuous hemoglobin (SpHb) monitoring has absolute and trending accuracy similar to widely used invasive methods of hemoglobin measurement with additional advantages. Study highlights that "the ability to measure hemoglobin noninvasively and continuously with SpHb has significant potential to facilitate hemoglobin monitoring, hasten the detection of acute anemia, and avoid the complications, expense, and discomfort associated with invasive blood draws."1
Total hemoglobin measurement is one of the most frequently ordered laboratory tests. But, traditional blood analysis has many drawbacks, including complexity, time-consuming turnaround times that can impact clinical decisions, and blood loss due to invasive blood draws that have been found to contribute substantially to the anemic conditions that commonly occur in critically ill patients. Other studies suggest that blood loss from invasive blood draws induce iatrogenic anemia, with over 90% of intensive care unit (ICU) patients becoming anemic by the third day of ICU admission.2 In contrast, Masimo SpHb offers a completely noninvasive method of continuously measuring hemoglobin levels without removing a drop of blood. This study is the first to evaluate the performance and accuracy of SpHb against three invasive techniques commonly used in the ICU for measuring hemoglobin.
In the study, conducted at the University Hospital in Poitiers, France, researchers compared SpHb measurements (obtained noninvasively using Radical-7 version 7.6.0.1 and Adult rainbow ReSposable Sensors R2-25, Rev. E) to invasive laboratory measurements analyzed by a satellite lab CO-Oximeter (Siemens RapidPoint 405), a HemoCue point-of-care device, and a laboratory hematology analyzer-considered the reference device (Sysmex XT-2000i) in 62 ICU patients. Results showed bias and limits of agreement for SpHb were 0.0 +1.0 g/dL, 0.3 + 1.3 g/dL for HemoCue, and 0.9 + 0.6 g/dL for the satellite lab CO-Oximeter compared to the reference device. SpHb showed similar trend accuracy to the satellite lab CO-Oximeter, whereas the HemoCue point-of-care device did not appear to follow the trend of the laboratory analyzer or reference device. As a result of these findings, researchers concluded that SpHb "has absolute accuracy and trending accuracy similar to widely used invasive methods of hemoglobin measurement at bedside" and has "the additional advantages of providing continuous measurements, noninvasively, which may facilitate hemoglobin monitoring in the intensive care unit."
The researchers commented that although invasive CO-Oximeters are an accepted laboratory standard for assessing hemoglobin and have high interdevice reproducibility, SpHb "had the best accuracy as evidenced by the highest concordance co-efficient correlation and the lowest Arms."
SpHb is available as part of Masimo rainbow SET Pulse CO-Oximetry-a breakthrough technology platform that noninvasively and continuously measures multiple blood constituents and physiological parameters that previously required invasive procedures, including:
total hemoglobin (SpHb), oxygen content (SpOC), carboxyhemoglobin (SpCO), methemoglobin (SpMet), Pleth Variability Index (PVI), perfusion index (PI), and acoustic respiration rate (RRa), in addition to the 'gold standard' Measure-Through Motion and Low Perfusion performance of Masimo SET oxyhemoglobin (SpO2), and pulse rate (PR).
To see a summary of all known clinical studies and abstracts on Masimo SpHb, please visit:
https://professional.masimo.com/evidence/featured-studies/feature/.
1 Frasca D, Dahyot-Fizelier D, Catherine K, Levrat Q, Debaene B, Mimoz O. "Accuracy of a Continuous Noninvasive Hemoglobin Monitor in Intensive Care Unit Patients." Crit Care Med October 2011; Vol. 39, No. 10. For further reading, visit: http://journals.lww.com/ccmjournal/Abstract/2011/10000/Accuracy_of_a_continuous_noninvasive_hemoglobin.10.asp
2 Rodriguez RM, Corwin HL, Gettinger A, et al. "Nutritional Deficiencies and Blunted Erythropoietin Response as Causes of the Anemia of Critical Illness." J Crit Care 2001; 16:36-41. For further reading, visit: http://burndoc.net/nutrition_epogen.pdf
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb), oxygen content (SpOCTM), carboxyhemoglobin (SpCO), methemoglobin (SpMet), and Pleth Variability Index (PVI), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic MonitoringTM, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications" Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SpHb provides continuous monitoring of hemoglobin levels for all patients, and risks related to our assumptions that Masimo SpHb monitoring has the potential to decrease the frequency of invasive blood testing, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation.
The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


U.S. Health & Human Services Makes Critical Congenital Heart Defect Screening Using Motion-Tolerant Pulse Oximetry a Nationwide Newborn Screening Standard Implementation Strategy and Protocols Recommended by Federal Advisory Committee of Leading Associations (AAP, ACC, AHA, HHS) Along With Findings From New UK Study Using Masimo SET Support Motion-Tolerant, Low Perfusion Pulse Oximetry For Improved CHD Screening in Newborns
Irvine, California – September 23, 2011 – Masimo (NASDAQ: MASI) announced today that the U.S. Department of Health & Human Services (HHS) has added Critical Congenital Heart Defects (CCHD) screening using motion-tolerant pulse oximetry as a national newborn screening standard. In addition, the largest UK study of pulse oximetry screening for CHD detection, published online in the Lancet (appearing in the November 2011 print edition), demonstrates that when Masimo SET®Measure-Through Motion and Low Perfusion pulse oximetry was used to screen newborns before hospital discharge, it enabled clinicians to increase CHD detection and provided a cost-effective method for universal screening with high sensitivity.1
An International Problem—Undetected / Undiagnosed CHD in Newborns
Among the approximately 4.2 million babies born in the U.S. annually,2 CHD is the most prevalent form of birth defect and is the #1 cause of infant death.3 HHS estimates that approximately 7-9 babies per 1,000 live births have some form of CHD.3 Yet, up to 30% of infant deaths from CHD occurred before diagnosis4—leading many to question the effectiveness of current newborn screening methods. Current methods of CHD detection rely largely on newborn physical examination but fail to identify approximately 50% of CHD cases5—leaving many vulnerable newborns undiagnosed at hospital discharge.
The Strategy for a Nationwide Solution—"Motion-Tolerant Pulse Oximetry"
In a letter dated September 21, 2011, HHS Secretary Kathleen Sebelius outlined the decision to adopt expert panel recommendations for universal CCHD screening by pulse oximetry for all newborns into federal Recommended Uniform Screening Panel (RUSP) Guidelines—the national newborn screening system standards and policies. Citing the "emerging evidence base for the utility of early diagnosis and detection of CCHD via measurement of blood oxygen saturation" and the "momentum and commitment" at the state and federal levels," Sebelius directed federal agencies to "proceed expeditiously with implementation." The federal Agency Plan of Action outlined by HHS calls for the National Institutes of Health (NIH) to fund technology, process, care, and outcomes research activities; the Centers for Disease Control and Prevention (CDC) to fund surveillance activities to monitor infant morbidity and mortality outcomes; and the Health Resources and Services Administration (HRSA) to guide development of screening standards and implementation infrastructure, including training materials.3
In August 2011, a panel of pediatric and cardiac experts from the American Academy of Pediatrics (AAP), the American College of Cardiology (ACC), and the American Heart Association (AHA), in conjunction with the HHS Secretary's Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC), acted on the HHS 2010 recommendation6 and outlined a strategy for routine screening of newborns to improve detection of CHD.7 The 28-page report recommends that newborn screening be done with "motion-tolerant pulse oximeters that report functional oxygen saturation, have been validated in low perfusion conditions, have been cleared by the FDA for use in newborns, and have a 2% root-mean-square accuracy." The report also outlined a 5-point implementation strategy and follow-up procedures, which includes screening, diagnostic confirmation, electronic results reporting, primary care follow-up, surveillance and tracking.
Gerard R. Martin, M.D., F.A.A.P., F.A.C.C., Senior Vice President of the Center for Heart, Lung and Kidney Disease at Children's National Medical Center in Washington, D.C., stated, "The excellent results that we can now achieve in correcting critical congenital heart defects make timely diagnosis even more important. Adding pulse oximetry screening as an important adjunct to fetal ultrasound and newborn physical examination will help to ensure that no baby is missed. This is major win for babies born with congenital heart diseases, as well as the families and providers who care for them."
Latest Published Study to Show Masimo SET Improves Early CHD Detection in Newborns
In the current Lancet-published study, researchers screened 20,055 asymptomatic newborn babies (gestation >34 weeks) at six UK maternity units using Masimo SET pulse oximetry before discharge. Newborns with oxygen saturation (SpO2) of less than 95% in either limb or a difference of more than 2% between limbs were considered positive for CHD and underwent echocardiography. Results showed that 53 babies had major CHDs (24 critical)—a prevalence of 2.6 per 1,000 live births. Masimo SET pulse oximetry achieved sensitivity of 75% (95% CI 53·29–90·23) for critical CHDs (causing death or requiring invasive intervention before 28 days) and 49% (95% CI 35·06–63·16) for all major CHDs (causing death or requiring invasive intervention within 12 months of age.)1
Findings also showed that Masimo SET pulse oximetry helped clinicians increase CHD detection by 34% versus antenatal ultrasonography alone (from 35 cases to 53 cases). In addition, out of the 0.8% false-positive results (169), 27% had other problems that required medical intervention (6 were significant but not major congenital heart defects and 40 were other illnesses like respiratory disorders and infections)—showing that the sensitivity of Masimo SET enabled identification of other life-threatening newborn conditions that likely would have otherwise gone undiagnosed. Researchers concluded that: "Pulse oximetry is a safe, feasible test that adds value to existing screening. It identifies cases of critical congenital heart defects that go undetected with antenatal ultrasonography. The early detection of other diseases is an additional advantage."
According to Dr. Mitchell Goldstein, Neonatologist and Associate Professor of Pediatrics at Loma Linda University Children's Hospital in California, stated, "Congenital heart defects are difficult to detect because many newborns do not show outward signs of failure until they decompensate. Today, congenital heart disease screening by Masimo SET pulse oximetry represents a safe and inexpensive way to screen for heart disease before the baby has a life-threatening event. Federal recommendations for universal screening underscore the critical importance of what SET has done to make pulse oximetry a clinically useful tool."
Other Studies Prove: Pulse Oximetry Technology Matters
In addition to the current Lancet-published study, two of the largest CHD studies conducted to date have also shown that Masimo SET is effective in screening newborns for CHD, including a Swedish study of 39,821 newborns published in the British Medical Journal8 and a Norwegian study of 50,008 newborns published in the Journal of Pediatrics.9
Masimo SET (Signal Extraction Technology) is clinically proven in over 100 independent and objective studies to provide the most accurate and reliable SpO2 and pulse rate measurements, even under the most challenging conditions of patient motion and low peripheral perfusion. Utilizing patented signal processing technologies—including parallel engines and adaptive filters—to deliver accurate and reliable measurements of a patient's true oxygenation status, Masimo SET has been shown to reduce false patient monitor alarms by over 95% and expand true alarm detection to over 97%. Because of this superior performance, Masimo SET is the leading Measure-Through Motion and Low Perfusion SpO2 solution incorporated into over 100 multiparameter monitors and 50 monitoring brands—including Atom, Datascope, GE Medical, Medtronic, Philips, Spacelabs, Welch Allyn, and Zoll, among others.
Joe Kiani, Founder, Chairman, and CEO of Masimo, commented that, "We commend HHS, the federal advisory committee, individual states, and the countless medical professional, public health leaders, and advocates that worked so hard to give our nation's smallest and most vulnerable patients a resounding voice. Adding CHD screening by Measure-Through Motion and Low Perfusion pulse oximetry is a significant advancement of our nation's newborn screening standard that will save untold newborn lives and help to prevent the agony of families who are left to cope with the devastating effects of undetected CHD."
To date, because of the overwhelming clinical evidence and appeals by grassroots advocates made up largely of parents with babies affected by CHD, Maryland and New Jersey have passed legislation promoting CHD screening by pulse oximetry in newborns with similar screening initiatives taking place in Beijing, China and the U.K. For a complete list of states' status on CHD screening legislation, visit: www.cchdscreeningmap.com
* Note: Masimo SET technology is not indicated to diagnose or treat congenital heart defects.
Editor's note:
References:
1. Andrew K Ewer, Lee J Middleton, Alexandra T Furmston, Abhay Bhoyar, Jane P Daniels, Shakila Thangaratinam, Jonathan J Deeks, Khalid S Khan. "Pulse oximetry screening for congenital heart defects in newborn infants (PulseOx): a test accuracy study." The Lancet 2011: Vol. 378; No. 9793; pp 785-794. For further reading visit: http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(11)60753-8/fulltext#article_upsell.
2. Sutton, PD. "Recent trends in births and fertility rates through June 2010." National Center for Health Statistics Health E-Stat 2010. Available at: http://www.cdc.gov/nchs/data/hestat/births2010/births2010.htm
3. Secretary of Health & Human Services' letter to the Secretary's Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC); dated September 21, 2011. For further reading visit: http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/recommendations/correspondence/cyanoticheartsecre09212011.pdf.
4. De-Wahl Granelli A, Mellander M, Sunnegardh J, Sandberg K, Östman -Smith I. "Screening for duct-dependent congenital heart disease with pulse oximetry: a critical evaluation of strategies to maximize sensitivity." Acta Paediatr 2005;94:1590-6. For further reading visit: http://onlinelibrary.wiley.com/doi/10.1111/j.1651-2227.2005.tb01834.x/abstract;jsessionid=E0F922B8E4E2950354BB77D36463ED96.d01t03?systemMessage=Wiley+Online+Library+will+be+disrupted+3+Sep+from+10-12+BST+for+monthly+maintenance.
5. Wren C, Richmond S, Donaldson L. "Presentation of congenital heart disease in infancy: implications for routine examination." Arch Dis Child Fetal Neonatal Ed. 1999;80:F49–F53. For further reading visit: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1720871/.
6. Secretary's Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC); Recommendation Letter to HHS Secretary Kathleen Sebelius; October 15, 2010. For further reading visit: http://www.hrsa.gov/heritabledisorderscommittee/correspondence/HowellLettertoSebeliusOct152010.pdf. Secretary of Health and Human Services; Letter to SACHDNC on CHD Screening Recommendation; April 20, 2011. For further reading visit: http://www.hrsa.gov/heritabledisorderscommittee/correspondence/CCCHDSecResponse042011.pdf.
7. Alex R. Kemper, William T. Mahle, Gerard R. Martin, W. Carl Cooley, Praveen Kumar, W. Robert Morrow, Kellie Kelm, Gail D. Pearson, Jill Glidewell, Scott D. Grosse, R. Rodney Howell. "Strategies for Implementing Screening for Critical Congenital Heart Disease." Pediatrics; published online ahead of print Aug. 22, 2011. For further reading visit: http://pediatrics.aappublications.org/site/misc/2011-1317.preprint.pdf.
8. Anne de-Wahl Granelli, Margareta Wennergren, Kenneth Sandberg, Mats Mellander, Carina Bejlum, Leif Inganäs, Monica Eriksson, Niklas Segerdahl, Annelie Ågren, Britt-Marie Ekman-Joelsson, Jan Sunnegårdh, Mario Verdicchio, Ingegerd Östman-Smith. "Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39 821 newborns." British Medical Journal (BMJ) January 2009; 338:a3037. For further reading visit: http://www.bmj.com/content/338/bmj.a3037.full.
9. Meberg A, Brugmann-Pieper S., Due R. et al. "First Day of Life Pulse Oximetry Screening to Detect Congenital Heart Defects." Journal of Pediatrics 2008: 761-765. For further reading visit: http://www.ncbi.nlm.nih.gov/pubmed/18492511.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo SET improves CHD detection in newborns before hospital discharge, risks related to our assumptions of the repeatability of clinical results obtained, and risks related to our assumptions that Masimo SET pulse oximetry technology is a superior solution for CHD detection and newborn screening applications, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.

Centre Hospitalier de Chaumont in France Implements State-of-the-Art
Remote Monitoring System to Advance Patient Safety
Masimo Patient SafetyNet™ Provides a New Level of Safety and
Peace of Mind for Hospitalized Patients
Paris, France & Irvine, California – September 12, 2011 – Patients admitted to Centre Hospitalier (CH) de Chaumont in France can feel safer thanks to a new technological guardian angel. Today, in addition to hospital staff, patients at Chaumont are being safeguarded by Masimo Patient SafetyNet™—a new, advanced remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable patient deaths and injuries associated with failure to rescue events—one of today's most common medical errors.1
"Patients need to know that there are systems dedicated specifically to safeguarding their health and safety in place at some hospitals, and we're on the forefront of this effort," stated Vartan Iknoyan, Biomedical Chief Engineer at Centre Hospitalier de Chaumont. "As a Patient SafetyNet™ hospital, Chaumont now utilizes this state-of-the-art remote noninvasive monitoring system to help keep our patients from deteriorating to the point of critical danger—helping us to achieve better outcomes, recoveries, and patient satisfaction in the process."
Developed by Masimo (NASDAQ: MASI), Patient SafetyNet™ helps keep patients safer by noninvasively, continuously measuring and tracking the underlying physiological condition of up to 80 patients on four floors to detect real-time changes and abnormalities that signal declining health status. The moment a patient's condition deteriorates, the system automatically sends wireless alerts directly to the pager or smartphone of assigned clinicians—prompting an immediate, potentially lifesaving response at the patient's bedside. Patient SafetyNet™ has been clinically-proven to significantly decrease traumatic critical events by 65% and costly ICU transfers by 48% to improve patient outcomes and reduce costs.1
According to Dr. Bernard Simon, Head of the Department of Pneumology and Oncology at Centre Hospitalier de Chaumont, Patient SafetyNet has made a big impact on care. "First thing we noticed was a significant reduction in false alarms because the technology is more advanced and accurate. Not having to chase down false alarms has allowed our clinicians to be more efficient and focus more attention on the care and treatment of patients. The system's ability to monitor and detect real-time changes in a patient's condition enable us to anticipate serious problems and intervene earlier, which allows us to reduce critical events, transfers to the ICU, and length of stays by improving clinical decision-making so that our patients can get better sooner."
The Patient SafetyNet™ system features a noninvasive patient sensor [finger or acoustic respiration neck sensor] and bedside patient monitor [Masimo Rad-87®or Radical-7®Pulse CO-Oximeter™] that continuously captures vital physiological measurements—including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), acoustic respiration rate (RRa™), oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR)—and wirelessly transmits them to the
central monitoring station, which displays the real-time clinical status of all connected patients and instantly routes alarms to the pager or smartphone of assigned clinicians. (See Figure 1 for the Complete System)1 Taenzer AH, Pyke JB, McGrath SP, Blike GT. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study" Anesthesiology; February 2010; Vol 112, Issue 2, pp 282-287.
About Chaumont Hospital
The origin of the original hospital goes back to the beginning of this millenium, but closed its doors in 1748 for maladjustment and insalubrity. Donations allowed the startup, in 1767, of the hospital of Chaumont. Under the material and spiritual control of the Brotherhood of the Girls of Charity, the hospital increased in size during the 19th century, was destroyed and then replaced by part of the new technical wing sheltering the laboratory (Villemin Pavilion), the urgent care reception and the SAMU/SMUR. Then right before the First World War, the MAILLOT and LARREY wings were established and reserved for the hospitalization of soldiers.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic MonitoringTM, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo Patient SafetyNet can help keep patients safer by noninvasively, continuously measuring and tracking their underlying physiological condition to help hospitals avoid preventable patient deaths and injuries associated with failure to rescue events, risks related to our assumptions of the repeatability of clinical results obtained, and risks related to the system's ability to significantly decrease traumatic critical events and costly ICU transfers to help improve patient outcomes and reduce costs, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Dana Banks
Masimo Corporation
Director of Corporate Communications
+ (949) 297-7348
dbanks@masimo.com
Audrey Valckenaere
Centre Hospitalier de Chaumont
(33) 03 25 30 71 49
Audrey.valckenaere@ch-chaumont.fr
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Shriners Hospitals for Children®- Honolulu: First in Hawaii to Implement Masimo Noninvasive Hemoglobin Monitoring Technology
Technology Reliably Monitors Hemoglobin Levels without Needles Masimo Noninvasive and Continuous Hemoglobin (SpHb®) Helps Hospital Reduce the Risk of Undetected Bleeding and Unnecessary Blood Transfusions to Improve Patient Safety
 Honolulu, Hawaii & Irvine, California – August 31, 2011 – The healthcare professionals at Shriners Hospitals for Children®- Honolulu, which perform hundreds of orthopaedic surgeries on children each year, are using the latest in medical technology from Masimo (NASDAQ: MASI) to noninvasively monitor patient blood levels during selected surgeries. Without the use of needles, the innovative technology instantly lets the healthcare team know if a patient has internal bleeding so they can initiate a lifesaving blood transfusion the moment one is needed. Masimo noninvasive and continuous hemoglobin (SpHb®)monitoring technology is also helping the nonprofit Honolulu hospital save precious time and money associated with lab results and unnecessary blood transfusions.
Honolulu, Hawaii & Irvine, California – August 31, 2011 – The healthcare professionals at Shriners Hospitals for Children®- Honolulu, which perform hundreds of orthopaedic surgeries on children each year, are using the latest in medical technology from Masimo (NASDAQ: MASI) to noninvasively monitor patient blood levels during selected surgeries. Without the use of needles, the innovative technology instantly lets the healthcare team know if a patient has internal bleeding so they can initiate a lifesaving blood transfusion the moment one is needed. Masimo noninvasive and continuous hemoglobin (SpHb®)monitoring technology is also helping the nonprofit Honolulu hospital save precious time and money associated with lab results and unnecessary blood transfusions.
"Knowing whether a patient's hemoglobin blood level is improving or falling to a critical point where a blood transfusion is necessary provides a good outcome for our young patients," said Harlan Klein, MD, Chief of Anesthesiology at Shriners Hospitals for Children®- Honolulu, who monitors vital signs of patients during surgeries.
According to National Institutes of Health (NIH) estimates, about five million patients receive blood transfusions each year in the United States, and more than halfof heart surgery patients need at least one transfusion of red blood cells.1 Blood transfusions, which replenish blood lost during surgeries, can be lifesaving, but they can also cause life-threatening complications. Blood transfusions can improve outcomes only when used in the right patient, at the right time, and in the right dose. Masimo SpHb allows healthcare professionals to accurately and reliably track hemoglobin changes occurring in real-time—providing earlier indications of directional changes than intermittent invasive hemoglobin values.2
|
Figure 1—Masimo Radical-7 |
"In the past, we've only received glimpses of our patients' hemoglobin levels from lab measurements, but now we have complete and real-time hemoglobin visibility," said Richard DiBucci, RN, Inpatient and Surgical Services Manager of the Honolulu hospital. "We not only can spot hemoglobin changes as they occur, but also can see where they are heading. This helps us to identify upward and downward hemoglobin trends on a second-by-second basis, which has been of tremendous value."
Masimo SpHb is part of Masimo rainbow®SET Pulse CO-Oximetry—a breakthrough technology platform that allows hospitals to noninvasively measure and continuously monitor blood constituents and physiological parameters that previously required invasive procedures—including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the 'gold-standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR). The oximetry standard-of-care at leading hospitals worldwide, Masimo rainbow®SET provides immediate real-time results that enable clinicians to more rapidly assess patients and detect and treat adverse, potentially life-threatening conditions earlier.
1 National Institutes of Health; NIH News; June 21, 2010; www.public.nhlbi.nih.gov/newsroom/home/GetPressRelease.aspx?id=2710
2 L. Lamhaut, R. Apriotesei, M. Lejay, B. Vivien, P. Carli. "Comparison Between a New Noninvasive Continuous Technology of Spectrophotometry-based and RBC Count for Haemoglobin Monitoring During Surgery with Hemorrhagic Risk" Eur J Anaesthesiol 2010; 27 (Suppl 47): 3AP7-1.
About Shriners Hospitals for Children®- Honolulu
Shriners Hospitals for Children®– Honolulu provides innovative pediatric orthopaedic care to children in Hawaii and throughout the Pacific Basin. There are two qualifications for care at Shriners Hospital: Children must be under age 18 and have an orthopaedic condition that can be treated. All care at the hospital is provided to a child, regardless of the patient's family ability to pay. More than 28,000 children have received orthopaedic care at the Honolulu hospital since it opened in 1923. There are 22 Shriners Hospitals in the United States, Canada and Mexico providing advanced care for children with orthopaedic conditions, burns, spinal cord injuries, and cleft lip and palate.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Contact:
Nathan Hokama
Strategic Communication Solutions
(808) 226-7470
Dana Banks
Masimo Corporation
(949) 297-7348
(949) 309-7759 (mobile)
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Pronto-7™ Receives Japanese and Canadian Regulatory Clearances
Irvine, California – August 29, 2011 – Masimo (NASDAQ: MASI) today announced the Japanese Ministry of Health Labor & Welfare (MHLW) and Health Canada regulatory clearances of Masimo Pronto-7™— enabling clinicians throughout Japan and Canada to quickly and conveniently measure total hemoglobin (SpHb®), SpO2, perfusion index, and pulse rate without removing a drop of blood.
Hemoglobin is one of the most commonly ordered tests in both the hospital and pre-hospital settings because it is critical to assessing blood loss following trauma, during surgery, and during hospital stay, as well as a patient's need for a blood transfusion. However, traditional lab testing requires a painful needle stick for the patient, time-consuming blood draws for the clinician, and typically provides delayed results. Masimo SpHb provides immediate real-time hemoglobin results that enable clinicians to more rapidly assess patients and detect and treat internal bleeding and low hemoglobin conditions earlier. And, although Masimo noninvasive and continuous total hemoglobin (SpHb) monitoring—commercially available in both Japan and Canada for over a year—is the preferred technology for hospitals and inpatient care centers, access to quick and easy spot-check hemoglobin measurements are valuable for a variety of healthcare assessment applications, including physician offices, outpatient care centers, and pre-hospital emergency settings.
Pronto-7 Makes Noninvasive Hemoglobin Testing a Reality in Japan and Canada Japanese and Canadian regulatory clearances for the palm-sized, handheld Pronto-7 and noninvasive finger sensors make it possible for clinicians to take the pain and wait out of traditional hemoglobin blood testing. The Pronto-7 offers a breakthrough solution for measuring total hemoglobin, SpO2, perfusion index, and pulse rate in less than one minute without the needles, time-consuming laboratory analysis, risk of blood contamination, hazardous medical waste, and patient discomfort associated with traditional blood tests. With dimensions of just 13 cm x 7.2 cm x 2.5 cm (5.1" x 2.8" x 1") and weight of 296 grams (10.5 ounces), the palm-sized Pronto-7 puts the power of noninvasive hemoglobin spot-check testing, along with SpO2, perfusion index, and pulse rate into any clinician's hands in various clinical settings—including physician offices, hospitals, clinics, and on the scene of medical emergencies.
Japanese and Canadian regulatory clearances for the palm-sized, handheld Pronto-7 and noninvasive finger sensors make it possible for clinicians to take the pain and wait out of traditional hemoglobin blood testing. The Pronto-7 offers a breakthrough solution for measuring total hemoglobin, SpO2, perfusion index, and pulse rate in less than one minute without the needles, time-consuming laboratory analysis, risk of blood contamination, hazardous medical waste, and patient discomfort associated with traditional blood tests. With dimensions of just 13 cm x 7.2 cm x 2.5 cm (5.1" x 2.8" x 1") and weight of 296 grams (10.5 ounces), the palm-sized Pronto-7 puts the power of noninvasive hemoglobin spot-check testing, along with SpO2, perfusion index, and pulse rate into any clinician's hands in various clinical settings—including physician offices, hospitals, clinics, and on the scene of medical emergencies.
In addition to Normal Mode, Pronto-7 offers Max Sensitivity Mode that allows measurement over a broader range of patients and three noninvasive sensor sizes to accommodate the range of finger diameters. Each sensor is color-coded to make size identification easy (Small-Yellow, Medium-Red, and Large-White) and optimized to improve performance in low ambient temperatures. And, because of the device's embedded 802.11 b/g and Bluetooth communication capability, wireless printing or emailing of test results is enabled with future upgrades that will allow for wireless transmission to electronic health record (EHR) systems.



Prior to the introduction of the Pronto-7, Masimo received U.S. FDA 510(k) and CE Mark clearance and first introduced the ability to noninvasively and continuously measure total hemoglobin (SpHb) in 2008 using its Radical-7 bedside Pulse CO-Oximeter. In 2009, Masimo launched Pronto®—its first handheld noninvasive spot-check device for total hemoglobin, SpO2, perfusion index, and pulse rate measurements. And in 2010, Masimo established Medicare reimbursement for transcutaneous hemoglobin measurement in the United States.
Masimo Founder and CEO, Joe Kiani, stated: "The potential impact of noninvasive total hemoglobin, SpO2, perfusion index, and pulse rate spot-check measurement offers healthcare providers in Japan and Canada the opportunity to transform the way and amount of time it takes to deliver the right care, at the right time to patients. We believe that the Pronto-7 will help clinicians to better assess more patients and make better clinical decisions based on the availability of quick and noninvasive hemoglobin measurement."
* Masimo Pronto-7 is pending FDA 510(k) clearance in the U.S.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using the enhanced Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at Morgan Stanley Global Healthcare Conference
IRVINE, Calif., August 29, 2011 — Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the Morgan Stanley Global Healthcare Conference at the Grand Hyatt New York on Tuesday, September 13, 2011, at 1:35 p.m. Eastern Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Obtains CE Mark for SEDLine, Launches EEG-Based Brain Function Monitors in European Market
European Availability Expands Access to Critical Care Brain Function Platform
Irvine, California – August 24, 2011 – Masimo (NASDAQ: MASI) announced today that it has received CE Mark for its SEDLine brain function monitor—expanding access to and availability of SEDLine's advanced neuromonitoring technology to hospitals and clinicians across Europe. The only brain function monitor with four separate EEG channels in a single integrated algorithm SEDLine represents a critical advancement for the practice of anesthesia in Europe—more complete data for a more complete picture of brain function and sedation.
SEDLine expands the scope of real-time data and improves the management of an anesthetic case by enabling more individualized titration. Four channels of high-quality EEG data provide information about both sides of the brain to facilitate immediate detection of asymmetrical activity and yields a single sophisticated algorithm to give accurate, reliable information about a patient's response to anesthesia. Masimo's SEDLine Patient State Index (PSI™), a calculated measure of brain activity that reflects the patient's current level of sedation/anesthesia1, provides additional data to enhance anesthetic control and facilitate rapid assessment. With demonstrated reliability under challenging clinical conditions and superior resistance to cautery2, SEDLine offers a cost-effective solution for brain function monitoring that helps clinicians achieve targeted sedation throughout all phases of anesthesia—in the OR and ICU.
 Caption (top): SEDLine's 4-channels of high-quality EEG data provide information on both sides of the brain.Caption (bottom): Four active leads in the SEDLine sensor collect a higher volume of data in key areas of the frontal lobe. |
|---|
According to Michael Ramsay, M.D., Chief of Anesthesiology and Pain Management & President of the Research Institute at Baylor University Medical Center in Dallas, Texas, "For many of the liver transplants and cardiac anesthesia cases I participate in, SEDLine provides me with real-time access to more comprehensive brain activity data, which is especially critical when a surgical patient's hemodynamic parameters are rapidly changing. Because of SEDLine's unique four separate EEG channels in a single integrated algorithm—enabling greater control and sedation management—I believe it will also complement the use of Target Control Infusion narcotic based anesthesia, which is a widely used technique in Europe."
The introduction of EEG-based monitoring systems such as SEDLine to provide guided titration has been shown to reduce the amount of anesthetic used and decrease recovery time when used as an adjunct to current standard clinical practice.3-4 In fact, these studies show that SEDLine PSI facilitated more individualized titration of anesthesia that led to patients receiving significantly less anesthetic and recovering an average of 67% faster—ultimately enabling faster OR to PACU throughput with patients meeting discharge eligibility19% faster than non-SEDLine-monitored patients.
Masimo Founder and CEO, Joe Kiani, stated: "The risks associated with the expanded use of short-acting anesthetic drugs like Propofol, where patients go in and out of the sedation state very quickly, makes it increasingly important for anesthesiologists to maintain tighter control. Masimo's SEDLine PSI and our popular 4-channel waveforms help anesthesiologists keep their patients where they want them to be—improving sedation control and the management of anesthetized patients."
SEDLine is currently used by top U.S. hospitals, including Baylor Medical Center in Dallas, TX; Massachusetts General Hospital in Boston, MA; Stanford University Medical Center in Palo Alto, CA; New York Columbia Presbyterian Hospital in NYC; Loma Linda University Medical Center in Loma Linda, CA.
References:
1. Prichep LS, Gugino LD, John ER, et al. The Patient State Index as an indicator of the level of hypnosis under general anesthesia. Br J Anaesth. 2004,92:393-399. Available online at http://bja.oxfordjournals.org/cgi/content/full/92/3/393.
2. White PF, Tang J, Ma H, Wender RH, Sloninsky A, Kariger R. Is the Patient State Analyzer with the PSArray2 a cost-effective alternative to the Bispectral Index Monitor during the perioperative period? Anesth Analg. 2004;99:1429-1435.
3. Drover DR, Lemmens HJ, Pierce ET, et al. Patient State Index: titration of delivery and recovery from propofol, alfentanil, and nitrous oxide anesthesia.Anesthesiology. 2002;97:82-89.
4. Cassingham SF, Hebert T, Lemaire R, Cook R, McPherson PK. The Physiometrix PSA 4000 decreases propofol usage and hastens discharge in gynecological day surgery procedures. Anesthesiology. 2002;96:A5.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that SEDLine facilitates more individualized titration of anesthesia that leads to patients receiving significantly less anesthetic and recovering faster, risks related to our assumptions of the repeatability of clinical results obtained, and risks related to our assumptions that SEDLINE will facilitate immediate detection of asymmetrical activity in all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Announces Adoption of Stock Repurchase Program
Irvine, California – August 9, 2011 – Masimo (NASDAQ: MASI) today announced that its Board of Directors has authorized the repurchase of up to 3 million shares of the company's common stock. The stock repurchase program may be carried out at the direction of the company through open market purchases, block trades, and in privately negotiated transactions. The repurchase program will become effective on August 12, 2011 and is expected to continue for a period of 24 months unless it is terminated earlier by the Board of Directors. Any repurchases will be subject to the availability of stock, general market conditions, the trading price of the stock, alternative uses for capital and the company's financial performance. The company expects to fund the stock repurchase program through its available cash.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said: "This stock repurchase program underscores our continued belief and optimism in Masimo's long-term growth prospects, as well as our commitment to return value to our stockholders. Our management team and Board of Directors have a strong conviction in our growth prospects, business model and cash flow generation capability, and we believe that the stock represents an attractive value relative to our future outlook."
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements, including statements that the company will generate future cash flow, the repurchase of company stock constitutes an opportunity to increase stockholder value, available cash should provide sufficient liquidity to support the stock repurchase program, and the repurchase of our stock represents an attractive investment. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce the anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws. The repurchase program authorization does not require the company to purchase a specific number of shares and the program may be modified, suspended or terminated at any time. The timing and number of shares repurchased, if any, pursuant to the stock repurchase authorization will be subject to a number of factors, including current market conditions, legal constraints and available cash or other sources of funding. This press release is not an offer to purchase any securities.
Investor Contact:
Sheree Aronson
Vice President,
Investor Relations, Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Director,
Public Relations, Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation


Masimo Reports Second Quarter 2011 Financial Results
Irvine, California, August 9, 2011 – Masimo (NASDAQ: MASI) today announced its financial results for the second quarter ended July 2, 2011.
Q2 2011 Highlights (compared to Q2 2010):
- Total revenue, including royalties, rose 9% to $109.6 million
- Product revenue rose 17% to $102.6 million
- Masimo SET®and Masimo rainbow®SET unit shipments rose 2% to 37,300
- Masimo rainbow revenue rose 26% to $9.1 million
- GAAP EPS was $0.28 versus $0.24 in the prior period, which included $0.01 in one-time expenses. Excluding the prior period one-time items, GAAP EPS rose 12% from an adjusted $0.25 in Q2 2010
Masimo's total revenue, including royalties, for the second quarter rose 9% to $109.6 million, compared to $100.1 million for the second quarter of 2010. Masimo's second quarter product revenue rose 17% to $102.6 million, compared to $88.0 million for the second quarter of 2010. Revenue from Masimo rainbow products rose 26% to $9.1 million in the second quarter, compared to $7.2 million for the second quarter of 2010.
Net income for the second quarter was $17.0 million, or $0.28 per diluted share, compared to reported net income of $14.3 million, or $0.24 per diluted share, in the second quarter of 2010, which included $0.01 per diluted share in one-time marketing-related expenses. Excluding these one-time expenses, diluted earnings per share rose 12% in the second quarter of 2011 from the year-ago period.
During the second quarter, the company shipped approximately 37,300 Masimo SET pulse oximetry and Masimo rainbow SET Pulse CO-Oximetry units, excluding handheld units, up 2% compared to approximately 36,700 in the same prior year period. Masimo estimates its worldwide installed base as of July 2, 2011 to be 922,000 units, up 17% from 789,000 units as of July 3, 2010.
As of July 2, 2011, cash and cash equivalents was $125.7 million, compared to $88.3 million as of January 1, 2011.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 78780959. After the live webcast, the call will be available on Masimo's website through September 9, 2011. In addition, a telephonic replay of the call will be available through August 16, 2011. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 78780959.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about: our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies; and global demand for our technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact:
Sheree Aronson
Vice President,
Investor Relations, Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Director,
Public Relations, Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.



Masimo Introduces Pronto-7™ Internationally and Lifts Voluntary Recall
 |
|
The palm-sized Pronto-7, with dimensions of just 13 cm x 7.2 cm x 2.5 cm (5.1" x 2.8" x 1") and weight of 296 grams (10.5 ounces), offers a breakthrough solution for measuring hemoglobin, SpO2, pulse rate, and perfusion index in less than one minute—without the needles, time-consuming laboratory analysis, risk of blood contamination, hazardous medical waste, and patient discomfort associated with traditional blood tests. |
Irvine, California – August 8, 2011 – Masimo (NASDAQ: MASI) announced today that it has lifted the voluntary product recall the company imposed on the noninvasive finger sensor of its Pronto-7™ handheld device to improve the product's performance in low ambient temperatures. The Pronto-7 and new finger sensors are pending FDA 510(k) clearance in the U.S.
The palm-sized Pronto-7, with dimensions of just 13 cm x 7.2 cm x 2.5 cm (5.1" x 2.8" x 1") and weight of 296 grams (10.5 ounces), offers a breakthrough solution for measuring hemoglobin, SpO2, pulse rate, and perfusion index in less than one minute—without the needles, time-consuming laboratory analysis, risk of blood contamination, hazardous medical waste, and patient discomfort associated with traditional blood tests.
New product features and enhancements include the addition of Max Sensitivity Mode that allows measurement over a broader range of patients. The addition of three noninvasive sensor sizes permits the instrument to be used on a broader range of finger diameters. Each of the new sensors is color-coded to make size identification easy (Small-Yellow, Medium-Red, and Large-White).


Masimo Founder and CEO, Joe Kiani, stated: "We take the integrity of our design and our promises to our customers seriously. We worked through the recall until we had redesigned the sensor for Pronto-7 so that it would perform as intended in the broadest range of likely ambient temperatures. We are delighted to re-introduce the Pronto-7 and with it the potential impact of noninvasive hemoglobin spot-check measurements that offer healthcare providers around the world the opportunity to transform the way and amount of time it takes to deliver the right care, at the right time to patients. We believe that Pronto-7's latest features and enhancements will help clinicians to better assess more patients and make better clinical decisions based on the availability of these quick and noninvasive hemoglobin measurements."
As part of Masimo's verification process, over 11,443 Pronto-7 measurements were performed on 1,445 subjects at 14 sites and compared to hemoglobin measurements from a venous blood sample analyzed on a hematology analyzer (Coulter Counter). This testing resulted in a product specification of 1.0 g/dL for normal sensitivity mode and 1.1 g/dL for max sensitivity mode. While not a regulatory requirement, prior to lifting the voluntary recall Masimo elected to perform additional testing on Pronto-7 devices and sensors produced at a Masimo manufacturing facility. This additional testing included 474 subjects in three outpatient clinic-type environments. The hemoglobin measurements from the Pronto-7 and a point of care device using capillary blood (Hemocue) were compared to hemoglobin measurements from a venous blood sample analyzed on a Coulter Counter. The Hemocue showed a bias of -0.1 g/dL and a standard deviation of 1.6 g/dL, while the Pronto-7 showed a bias of was -0.1 g/dL and a standard deviation of 1.1 g/dL. Based on the clinical results obtained and positive feedback from clinicians, Masimo has initiated international availability of Pronto-7 in Europe, Middle East, Africa, South America, and Asia (except for countries requiring clearance, such as Japan).
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic MonitoringTM, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that repositioning the thermistor solves prior temperature measurement problems, risks related to our assumptions of the repeatability of clinical results obtained using the enhanced Masimo Pronto-7 and noninvasive sensor sizes, risks related to our belief that the Pronto-7 enables quick and easy noninvasive spot-checking of hemoglobin (SpHb®), SpO2, pulse rate, and perfusion index at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Report Second Quarter 2011 Financial Results after Market Close on August 9, 2011
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., July 19, 2011 -- Masimo (NASDAQ: MASI) announced today that it will release second quarter financial results for the period ended July 2, 2011, after the market closes on August 9, 2011. The release and conference call date is approximately one week later than Masimo's traditional release timing due to executive calendar conflicts during the month of July.
The conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 78780959. After the live webcast, the call will be available on Masimo's website through September 9, 2011. In addition, a telephonic replay of the call will be available through August 23, 2011. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 78780959.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo and Dartmouth-Hitchcock Medical Center, First Cross-Industry Collaborative Honored with the Institute for Technology and Healthcare Clinical Application Award
The Association for the Advancement of Medical Instrumentation (AAMI) Foundation Award Honors the Clinical Application and Efficacy Excellence Demonstrated Through Implementation of Masimo Patient SafetyNet™
Irvine, California – June 27, 2011 – Masimo Corporation (NASDAQ: MASI) announced today that two of the industry's top patient safety collaborators—James Welch, VP of Patient Safety Initiatives at Masimo, and George Blike, Medical Director of Patient Safety Training at Dartmouth-Hitchcock Medical Center—are the recipients of the AAMI Foundation'sInstitute for Technology and Healthcare Clinical Application Award. Awarded each year to an individual or group who has applied innovative clinical engineering practices or principles to solve patient care problems through demonstrated clinical application and efficacy excellence, this is the first time in the association's history that the award was shared between two cross-industry collaborators—a medical technology innovator/developer (Masimo) and a patient care provider/hospital facility (Dartmouth-Hitchcock Medical Center).
Honored for their cross-industry collaboration in the implementation of Masimo Patient SafetyNet™ at Dartmouth-Hitchcock, Welch (Masimo) and Blike (Darmouth-Hitchcock) combined their high-tech and human-touch expertise to install the remote monitoring and wireless clinician notification system based on Masimo SET®Measure-Through Motion and Low Perfusion pulse oximetry monitoring that led to a "significant drop" in key clinical outcome measures—including 65% fewer rescue events, 48% fewer ICU transfers, and reduced annualized ICU time by 135 days.1
"James Welch (with Masimo) and George Blike (with Dartmouth-Hitchcock) exemplify the term effective collaboration," stated Mary Logan, President of the Association for the Advancement of Medical Instrumentation. "What is particularly powerful about this duo is that they have modeled for all of us what can be accomplished when you have an effective collaboration between clinical engineering and front-line clinicians, between industry and patient safety experts, and between technology developers and a clinician who knows how to assess in a clinical setting the impact of a technology solution to a vexing safety problem. We can all be grateful for the power of what they have accomplished here."
Designed to improve patient safety through continuous pulse oximetry monitoring, Patient SafetyNet keeps general care floor patients safer by continuously, noninvasively, and remotely monitoring multiple physiological parameters, including arterial oxygen saturation and pulse rate, and automatically alerting clinicians to changes that signal patient distress or deterioration via pager or phone. The system's alarm escalation process is an important feature that notifies additional clinicians if a life-threatening alarm persists—ensuring that alarms are escalated to other clinicians in the event the assigned primary clinician is busy or unresponsive. With the system in place, Dartmouth-Hitchcock clinicians receive a pager notification when a patient's condition is worsening—allowing them to intervene before the condition becomes critical and requires more acute levels of care. This is particularly important for post-surgical patients who are at increased risk of serious injury or death resulting from the respiratory depression effects of patient-controlled analgesia (PCA) and opioids used for sedation and pain management.
The AAMI Foundation Awards Committee performs rigorous reviews of candidates who have demonstrated outstanding service or accomplishment with a significant impact on a specific medical device or on medical instrumentation in general, a service that results in the development of an important new medical device, a distinctive contribution toward the improvement of the use or safety of a medical device, or critical contributions to the enhancement of patient care through medical instrumentation. Their recognition of the significant impact made by both Welch and Blike in the cross-industry collaboration to improve patient safety on the general care floor with Patient SafetyNet is proof-positive that the combination of high-tech medical technology expertise and high-touch clinical care expertise are a winning pair for patients.
The Foundation'sprimary mission is to serve public welfare and improve patient safety by fostering support and recognition for the advancement of medical technology. It does this through recognition awards for excellence, scholarships, global outreach, and the Medical Device Safety Council. The Foundation's Excellence Awards recognize individuals and groups for their superior work in medical instrumentation development, research, and applications that improve public health care safety, as well as humanitarian efforts that apply health technology to improve global human conditions.
1 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. Available online at: http://journals.lww.com/anesthesiology/Abstract/publishahead/Impact_of_Pulse_Oximetry_Surveillance_on_Rescue.99692.aspx
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using Masimo rainbow SET measurements, risks related to our belief that rainbow measurements can help clinicians more rapidly assess patients to detect adverse conditions earlier, including the early signs of hemodynamic instability, potentially life-threatening conditions, and internal bleeding, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


New Clinical Research Presented at the European Society of Anaesthesia Demonstrates the Accuracy and Utility of Masimo SpHb and PVI
Irvine, California – June 16, 2011 – Masimo Corporation (NASDAQ: MASI) announced today that multiple new clinical research studies presented this week at the European Society of Anaesthesiology (ESA) Annual Congress in Amsterdam add to the growing body of evidence that Masimo noninvasive and continuous total hemoglobin (SpHb®) and Pleth Variability Index (PVI®) are accurate and useful noninvasive and continuous measurements in a variety of patients.
Two studies, from researchers in Japan and Sweden, evaluated the accuracy and reliability of SpHb in both surgical and ED patients and found clinically acceptable correlations between noninvasive SpHb measurements and invasive lab values. Researchers at the Hamamatsu University School of Medicine in Japan analyzed 89 hemoglobin samples from 16 surgical patients and found a correlation coefficient between SpHb (obtained using the Masimo Radical-7 and R-125 rev C sensor) and invasive lab values of 0.8 with a bias of 0.65 g/dL and precision of 1.1 g/dL. Citing this as the first introduction of SpHb to Japanese patients, researchers concluded that "SpHb is reliable on Japanese patients." 1 Similarly, researchers at the Karolinska Institute in Stockholm, Sweden, compared SpHb measurements (obtained using the Masimo Radical-7 software version 7.6.0.1 and adult ReSposable sensor rev E) to invasive venous blood sampling (tHb) in 30 patients admitted to the emergency room and found a very low bias of -0.2 and standard deviation of 1g/dL, with limits of agreement of -2.2 to 1.8 g/dL. Citing "fairly good agreement between tHb and SpHb values across the entire measurement range," researchers concluded that "SpHb monitoring provides hemoglobin values with good agreement to laboratory analysis of blood."2
Two other studies, conducted in France and Japan, analyzed finger, ear, and forehead sites for PVI measurement and found that sites on the head enable the best measurement. Previous studies have shown that PVI, as measured at the finger site, can help clinicians assess fluid responsiveness and improve fluid management to decrease patient risk. Results showed that Masimo ear and forehead sensors provide the best accuracy for PVI measurement. At the Louis Pradel Hospital in France, researchers evaluated PVI measurements obtained from the ear, forehead, and finger (using the Masimo Radical-7 and LNOP Adt sensor) in 16 patients during ongoing surgery and found that PVI correlations to pulse pressure variations (PPV) were r=0.72 at the ear, r=0.60 at the forehead, and r=0.43 at the finger (all p<0.001). PVI forehead >14% and PVI ear >15% predicted a PPV > 10% with 73% sensitivity and 72% specificity, while PVI finger > 13% predicted a PPV > 10% with a sensitivity of 78% and a specificity of 44%—leading researchers to conclude that "ear and forehead provide best accuracy for PVI."3 In Japan, researchers at Kagoshima University tested the effects of surgical stimuli on PVI measurements at the finger and forehead (obtained using the Masimo Radical-7 software version 7.3.11 and LNOP Adt sensor) in nine mechanically ventilated surgical patients and found that forehead PVI & perfusion index (PI) remained stable before and after skin incision while PVI captured at the finger site were more sensitive to vasomotor tone. Researchers concluded that "PVI obtained from the forehead may be a more reliable predictor of fluid responsiveness under conditions of varying vasomotor tone."4
SpHb and PVI are available as part of Masimo rainbow SET®platform—the first-and-only technology to noninvasively and continuously measure total hemoglobin (SpHb®) , oxygen content (SpOC™), carboxyhemoglobin (SpCO®) , methemoglobin (SpMet®) , Pleth Variability Index (PVI®) , perfusion index (PI), and acoustic respiration rate (RRa™) , in addition to the 'gold standard' Measure-Through Motion and Low Perfusion performance of Masimo SET®oxyhemoglobin (SpO2), and pulse rate (PR).
To see a summary of all known clinical studies and abstracts on Masimo SpHb, please visit: https://professional.masimo.com/evidence/featured-studies/feature/.
The ESA 2011 schedule of clinical abstracts presented can be accessed online at http://www.euroanaesthesia.org/sitecore/Content/Congresses/Euroanaesthesia%202011/~/media/Congress/2011%20Amsterdam/Scientific%20Programme/2011_Abstract%20Sessions_Day%20by%20Day_11-05-2011.ashx.
1 Ishida C, Shiraishi Y., Mimuro S., Yu S., Katoh T., Sato S. "Accuracy of a Noninvasive Measurement of Hemoglobin Via Pulse CO-Oximetry in Japanese Population." Hamamatsu University School of Medicine, Department of Anesthesiology and Intensive Care, Hamamatsu, Japan.
2 Svensen C.H., Rodhe P., Lundstrom N., Berglund E., Sjostrand F. "Use of a Noninvasive Hemoglobin Monitor for Volume Kinetic Analysis in an Emergency Room Setting." Karolinska Institute,/Department of Clinical Science and Education, Department of Anesthesiology and Intensive Care, Stockholm, Sweden.
3 Pavlakovitch I., Desebbe O., Cannesson M., Bastien O., Lehot J.J. "What is the Best Site for Measuring the Pleth Variability Index (PVI) During a Surgery Procedure?" Hospices Civils de Lyon Hopital L Pradel, Department of Anesthesiology and Intensive Care, Bron-Lyon, France.
4 Matsunaga A., Kakihan Y., Kuniyoshi T., Kumemura M. Uchida Y., Kanmura Y. "Difference Between Pleth Variability Index Obtained from the Finger and Forehead." Kagoshima University, Department of Anesthesiology, Kagoshima, Japan.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using Masimo SpHb and PVI noninvasive measurements, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Announces Rainbow Agreement with Philips
Irvine, California June 15, 2011 – Masimo (NASDAQ: MASI) today announced that it has reached a long-term non-exclusive agreement under which Philips will integrate Masimo rainbow SET®technology into Philips' products. Masimo expects to begin diligent co-development projects with Philips, which should allow rainbow SET technology to be available in Philips' products worldwide.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our Rainbow agreement with Philips. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce the anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Pronto-7 Wins GOLD in the 2011 Medical Design Excellence Award Competition—the Premier Awards Program for the Medical Technology Community
 The palm-sized Masimo Pronto-7™ offers noninvasive hemoglobin, SpO2, pulse rate, and PI spot-check results. The palm-sized Masimo Pronto-7™ offers noninvasive hemoglobin, SpO2, pulse rate, and PI spot-check results. |  |
|---|
Irvine, California – June 9, 2011 – Masimo Corporation (NASDAQ: MASI) announced today that its Pronto-7™ handheld noninvasive hemoglobin, arterial oxygen saturation (SpO2), pulse rate, and perfusion index spot-check testing device has received the GOLD 2011 Medical Design Excellence Award—the highest award given in the general hospital devices and therapeutic products category. The 2011 Medical Design Excellence Award winners were honored yesterday at a presentation ceremony in New York City's Jacob K. Javits Convention Center, in conjunction with the Medical Design & Manufacturing (MD&M) East Conference and Exposition (www.MDMEast.com).
The Medical Design Excellence Awards competition (www.MDEAwards.com) is organized and presented by UBM Canon (Los Angeles) and is the only awards program that exclusively recognizes contributions and advances in the design of medical products. Entries are evaluated on the basis of their design and engineering features, including innovative use of materials, user related functions that improve healthcare delivery and change traditional medical attitudes or practices, features that provide enhanced benefits to the patient, and the ability of the product development team to overcome design and engineering challenges so that the product meets its clinical objectives. A comprehensive review of the entries was performed by an impartial, multidisciplinary panel of third-party jurors with expertise in biomedical engineering, human factors, industrial design, medicine, and diagnostics.
The Pronto-7 is truly an incredible breakthrough for clinical users. The palm-sized device was designed for quick-and-easy noninvasive hemoglobin (SpHb®) spot-check testing, along with SpO2, pulse rate, and perfusion index. Extremely lightweight at approximately 296g, the Pronto-7 delivers total hemoglobin measurement results—a physiological parameter that previously required an invasive blood draw. Offering needle-less and pain-free hemoglobin blood testing, the Pronto-7 is a cost-effective solution for immediate, on-the-spot hemoglobin testing that is raising the expectations of patients and clinicians alike to more advanced healthcare at the earliest moment of care.
"We are honored that the judges have selected Pronto-7 as a GOLD MDEA winning product," said Joe Kiani, Founder and CEO of Masimo. "Pronto-7 was designed to help overcome many of the challenges and risks associated with invasive hemoglobin sampling for both clinicians and patients—enabling fast, accurate, painless, needle- and risk-free total hemoglobin measurements."
The new Pronto-7 with small, medium, and large size finger sensors will soon be launched internationally and is pending FDA 510(k) Clearance in the U.S.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that Masimo Pronto-7 delivers noninvasive hemoglobin measurements in under one minute for all patients, and risks related to our belief that Pronto-7 provides needle-less and pain-free hemoglobin blood testing for clinicians in virtually any environment, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at William Blair & Company 31st Annual Growth Stock Conference
IRVINE, Calif., June 3, 2011 - Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the William Blair & Company 31st Annual Growth Stock Conference at the Four Seasons Hotel in Chicago on Wednesday, June 15, at 8:00 a.m. Central Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Initiates Limited Market Release of E1, Single-Patient-Use Ear Sensor for Pulse Oximetry Monitoring
Providing an Alternative Monitoring Site in the Ear Conchae for Faster Detection of Oxygen Saturation Changes
Irvine, California – June 2, 2011 – Masimo (NASDAQ: MASI) announced today FDA 510(k) Clearance, CE Mark, and limited market release of the industry's first single-patient-use ear sensor. Compared to digit sensors, the Masimo E1™ enables faster detection of oxygen saturation changes during low perfusion due to a variety of clinical factors, including sedative or medication-induced vasoconstriction.1,2,3 Compared to reusable ear sensors, it also avoids cross-contamination risks for patients and reduces the complexity of sensor management for clinicians, including cleaning, storage, and transport. In the limited market release, select clinicians around the world will have the opportunity to use and evaluate the performance and benefits of the E1 sensor.
Due to the signal processing limitations of conventional pulse oximetry during challenging conditions, clinicians often seek alternative monitoring sites on the head. In spite of the established need, available single-patient-use sensors for the head have suffered from unreliability. While Masimo SET Measure-through Motion and Low Perfusion pulse oximetry overcomes the limitations of conventional pulse oximetry and offers reliable true alarm detection and false alarm prevention during challenging conditions, there are still some advantages to monitoring on the head such as faster response to oxygenation changes during low perfusion and the use of an alternative site when the digit is unavailable.
 The Masimo E1™ Ear Sensor is placed conveniently and securely in the cavum conchae (inner aspect of the ear). |
|---|
As a single-patient-use ear sensor that is placed securely in the cavum conchae (the deep hollow near the ear canal opening), the E1 allows clinicians to combine Masimo SET performance with a reliable alternative monitoring site while minimizing cross-contamination. This site frees up the patient's hands and is easy-to-access for clinicians. Made of durable soft silicone, it has no moving parts that can entangle in the hair or irritate the skin. In addition to its advantages for faster detection of saturation changes during low perfusion, it also offers an alternative site when other monitoring sites are unavailable due to emergency transport or use by another type of head sensor such as those used for Masimo's SEDLine®brain function monitoring. In addition, by using the cavum conchae as the monitoring site, the E1 avoids the challenges associated with forehead sensors, including known limitations in the presence of venous pulsations and when patients are in a supine or trendelenburg position. When compared to the traditional ear lobe site, the cavum conchae site also offers better perfusion and superior signal quality—enabling improved monitoring performance in a sensor that stays in place more securely.
According to Dr. Daniel Davis, a contributor on the design of the E1 ear sensor, Professor of Clinical Emergency Medicine, and Director of the UCSD Center for Resuscitation Science in San Diego, California, "The Masimo E1 ear sensor is an ideal monitoring site solution for multiple clinical applications. With it, signal detection is optimized in low perfusion states (sepsis, hemorrhage, hypothermia) and in the presence of peripheral vascular disease. In my experience, the E1 ear sensor detected hypoxemia up to 2-3 minutes sooner, which is critically important during airway management, resuscitation, and inpatient apnea/hypopnea episodes. Additionally, the ear site is more accessible and offers distinct advantages when traditional peripheral monitoring sites are unavailable due to trauma or injury, are swollen and do not easily fit into a digital sensor, or are inaccessible due to drapes, bandages, and dressings. The E1 also eliminates the need for excess cords making it ideal for ambulatory and general care floor patients because it frees up their hands for better mobility, physical therapy, or exercise."
To locate a Masimo representative or distributor in your area and evaluate the E1 sensor, call 1-800-257-3810 or visit www.masimo.com.
1Tokuda K, Hayamizu K, Ogawa K, Hirai T, Irita K. "A Comparison of Finger, Ear and Forehead SpO2 on Detecting Oxygen Desaturation in Healthy Volunteers." Anesthesiology 2007; 107: A1544.
2 Redford D, Lichtenthal P, Barker SJ. "Evaluation of Masimo SET Ear and Forehead Pulse Oximetry and Nellcor MAX-FAST Forehead Pulse Oximetry." Anesthesiology 2004; 101: A593 and A579.
3 Whitman RA, Garrison ME, Oestreich TJ, Musumbi MS. "Evaluation of a New Reflectance Forehead Sensor in Detecting Oxygen Desaturation in Patients Undergoing Polysomnography." Anesthesiology 2003; 99: A553.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the Masimo E1 disposable ear sensor provides faster response times and rapid detection of oxygen saturation, risks related to our assumptions that the E1 sensor offers improved performance for patients in extremely low perfusion states, and risks related to our belief that the design of the E1 sensor does not put excessive pressure on the site and eliminates cross contamination risks, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at Goldman Sachs 32nd Annual Global Healthcare Conference
IRVINE, Calif., May 26, 2011 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the Goldman Sachs 32nd Annual Global Healthcare Conference at Terranea in Rancho Palos Verdes, California, on Thursday, June 9, 2011, at 8:40 a.m. Pacific Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Launches MS-2040, First Sub-45 mW Signal Extraction Pulse Oximetry Platform
New MS-2040 Circuit Board and External "Board-in-Cable" Promises to Usher in Breakthrough Pulse Oximetry Applications
Irvine, California – May 19, 2011 – Masimo (NASDAQ: MASI) announced today the launch of its new sub-45 milliwatt technology platform—MS-2040. This platform utilizes nearly a third of the power and half of the physical board size of previously available Signal Extraction Pulse Oximetry solutions while providing the same proven unprecedented Measure-Through Motion and Low Perfusion oxygen saturation (SpO2), pulse rate, and perfusion index (PI) measurement capabilities of Masimo SET®pulse oximetry, in addition to Pleth Variability Index (PVI).
  |
|---|
| CAPTION: The compact size and reduced power consumption of the Masimo MS-2040 double-stacked circuit board enables a broad range of reliable small, lightweight, handheld and wearable portable medical device possibilities. |
Using less than 45 milliwatts of power, Masimo MS-2040 boards are available in both a double-stack version (pictured) with dimensions of 1.8" x 1.15" x 0.46" and single-stack version with dimensions of 2.0" x 1.38" x 0.36". The small size and reduced power consumption of Masimo MS-2040 boards enhance integration into next-generation portable medical devices that need to be smaller, lighter, and more mobile—consuming less than a third of the power without sacrificing accuracy during motion and low perfusion.
According to Masimo President of Worldwide OEM Business and Corporate Development, Rick Fishel, "Over the last 15 years, Masimo has worked diligently to continuously enhance pulse oximetry features and performance, including the development of cutting-edge circuitry innovations that have reduced the size of our boards by 90% and power consumption by 98%. Reducing the power usage profile and physical size of our circuit boards enables Masimo and our OEM partners to design smaller, lighter, and more portable monitoring devices with the high performance pulse oximetry capabilities clinicians need for successful utilization in these new sites and applications."
The MS-2040 circuit board is also available as an external "board-in-cable" solution. Embedded within a completely self-contained patient cable, the "board-in-cable" solution provides external Masimo SET pulse oximetry capabilities for existing devices that do not presently offer an integrated pulse oximetry solution. This affords medical equipment manufacturers a simpler way to add Masimo SET pulse oximetry as an external (oximeter outside) option and enables a wide range of reliable handheld and patient-wearable application possibilities.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the high performance and low power consumption capabilities of the Masimo MS-2040 technology platform, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, MS-2040, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


New Published Study Demonstrates the Accuracy of Masimo Noninvasive and Continuous Hemoglobin Monitoring in Patients in Surgery and Intensive Care
Study Authors Indicate Masimo SpHb Allows for More Rapid and Appropriate Medical Interventions
Irvine, California – May 16, 2011 – Masimo (NASDAQ: MASI) announced today that findings from a new study, published in the American Journal of Surgery,demonstrate that noninvasive and continuous hemoglobin (SpHb®) monitoring correlates strongly with invasive laboratory hemoglobin testing and "can be beneficial in the operating room and critical care setting and may potentially decrease the invasiveness of both elective surgery and critical illness."1 This is the first study to examine the accuracy and reliability of Masimo SpHb for measuring hemoglobin and hemorrhage in both the operating room and intensive care unit (ICU).
Total hemoglobin measurement is one of the most frequently ordered laboratory tests. However, hemoglobin levels must be obtained through blood draws that are invasive, time-consuming, and can only provide data at one point in time. Since the introduction of pulse oximetry, monitoring of blood oxygenation in the operating room has become a standard of care. Today with the Masimo rainbow SET®platform, clinicians now have access to noninvasive and continuous pulse oximetry along with SpHb and other blood constituent parameters— all in one device and from one sensor.
In the study, conducted at Madigan Army Medical Center in Tacoma, Washington, researchers compared noninvasive SpHb and invasive laboratory measurements in a series of patients undergoing major surgical procedures in the operating room or with severe illnesses in the ICU. Using the Radical-7®Pulse CO-Oximeter, measurements were obtained on 25 elective surgical patients in the operating room and 45 critically ill patients in the ICU and compared to hemoglobin measurements obtained invasively every hour from the radial artery catheter. A total of 27 point-of-care invasive hemoglobin measurements (iStat®from Abbott) were also collected. Significant bleeding was identified in 44% of the surgical patients.
Results showed an overall correlation between SpHb and lab values of 0.78 (P < .001) with a mean difference of 0.15 g/dL (95% confidence interval, -0.03 to 0.32 g/dL). In the OR, the mean difference was 0.29 g/dL (95% confidence interval, 0.09 to 0.50g/dL) while in the ICU, the mean difference was 0.05 g/dL (95% confidence interval, 0.22 to 0.31 g/dL). In the five patients requiring intra-operative blood transfusion, there was a very strong correlation of 0.91 between SpHb and lab measurements. When the point-of-care measurements from iStat were compared to lab measurements, the results were similar to SpHb comparisons to lab measurements, with a correlation of 0.85 and a mean difference of 0.09 g/dL (95% confidence interval 0.17 to 0.34 g/dL).
As a result of these findings, researchers deemed that "noninvasive hemoglobin monitoring is especially important" for high-risk critically ill patients or patients undergoing high-risk operations, critically ill bleeding/transfused patients or patients undergoing operations with an expected high blood loss, and for patients not expected to have significant bleeding intra-operatively but in whom obtaining laboratory values would be difficult or impossible if not pre-planned.
Commenting that SpHb monitoring "will benefit the patient, intensivist, and anesthesiologist by allowing continuous physiological monitoring without having to occlude the arterial line and may even preclude the need for a central line when the purpose is only for frequent laboratory hemoglobin analysis," researchers concluded that "based on the strong correlation between SpHb and laboratory hemoglobin, noninvasive hemoglobin monitoring can be beneficial in the operating room and critical care setting and may potentially decrease the invasiveness of both elective surgery and critical illness while providing continuous hemoglobin monitoring in addition to standard pulse oximetry data."
SpHb is available as part of Masimo rainbow SET®platform—the first-and-only technology to noninvasively and continuously measure total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), perfusion index (PI), and acoustic respiration rate (RRa™), in addition to the 'gold standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), and pulse rate (PR).
1 Causey MW, Miller S, Foster A, Beekley A, Zenger D, Martin M. "Validation of noninvasive hemoglobin measurements using the Masimo Radical-7 SpHb Station." Am J Surgery 2011: 201: 590-596. For further reading, visit: http://www.americanjournalofsurgery.com/article/S0002-9610(11)00119-X/abstract.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET®Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SpHb is capable of providing continuous physiological monitoring of hemoglobin levels in all surgical or critically ill patients, and risks related to our assumptions that Masimo SpHb monitoring has the potential to decrease the invasiveness of elective surgery and critical illness, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Reports First Quarter 2011 Financial Results
Product revenue exceeds $100 million for first time in company's history
Q1 2011 Highlights (compared to Q1 2010):
- Total revenue, including royalties, rose 14% to $113.0 million
- Product revenue rose 18% to $101.6 million
- Masimo SET®and Masimo rainbow®SET unit shipments rose 16% to 43,100
- Masimo rainbow revenue rose 39% to $7.4 million
- GAAP EPS was $0.30 compared to $0.44 in Q1 2010, which included $0.20 in one-time items related to the antitrust lawsuit victory in Q1 2010. Excluding these one-time items, GAAP EPS of $0.30 was up 25% from an adjusted EPS of $0.24 in Q1 2010
Irvine, California, May 3, 2011 – Masimo (NASDAQ: MASI) today announced its financial results for the first quarter ended April 2, 2011.
Masimo's total revenue, including royalties, for the first quarter rose 14% to $113.0 million, compared to $98.8 million for the first quarter of 2010. Masimo's first quarter product revenue rose 18% to $101.6 million, compared to $85.9 million for the first quarter of 2010. Revenue from Masimo rainbow products rose 39% to $7.4 million in the first quarter, compared to $5.3 million for the first quarter of 2010.
Net income for the first quarter was $18.0 million, or $0.30 per diluted share, compared to reported net income of $26.7 million, or $0.44 per diluted share, in the first quarter of 2010 and compared to adjusted net income of $14.3 million, or $0.24 per diluted share, in the first quarter of 2010. Adjusted earnings per share in the first quarter of 2010 excludes $0.20 per diluted share from the net of a $30.1 million one-time gain related to an antitrust lawsuit victory and offset by $10.9 million in one-time grants and marketing initiatives. One-time grants in the first quarter of 2010 included a $10.3 million donation to establish the Masimo Foundation for Ethics, Innovation and Competition in Healthcare.
During the first quarter, the company shipped approximately 43,100 Masimo SET pulse oximetry and Masimo rainbow SET Pulse CO-Oximetry units, excluding handheld units, up 16% compared to approximately 37,100 in the same prior year period. Masimo estimates its worldwide installed base as of April 2, 2011 to be 890,000 units, up 18% from 757,000 units as of April 3, 2010.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "We continue to experience increasing global demand for our breakthrough technologies as evidenced by our new highs in product revenue and driver shipments in the quarter. We continue to pursue our mission to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications."
As of April 2, 2011, cash, cash equivalents and short-term investments totaled $105.6 million, compared to $88.3 million as of January 1, 2011
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 58223656. After the live webcast, the call will be available on Masimo's website through June 3, 2011. In addition, a telephonic replay of the call will be available through May 17, 2011. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 58223656.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about: our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies; and global demand for our technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations,
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.






Masimo Launches New Reusable SpCO® Sensor with Improved Accuracy and Reliability in Low Oxygen Saturation and Elevated Methemoglobin States
Irvine, California – April 28, 2011 – Masimo Corporation (NASDAQ: MASI) announced today the availability of new, enhanced versions of its reusable, noninvasive carboxyhemoglobin (SpCO) sensors—with improved accuracy and reliability performance for patients with low oxygen saturation (SpO2) between 90% and 95% and elevated methemoglobin (SpMet®) between 0% and 2%.
Low SpO2 and elevated SpMet states are known physiological factors that compromise SpCO accuracy and contribute to erroneous measurements. Although this limitation still applies, the new sensors will provide enhanced SpCO performance when a patient's functional SpO2 is > 90%, as well as when their SpMet is < 2%.
Carbon monoxide (CO) poisoning is a major cause of morbidity and mortality. At least 20,000 known exposures1 and 439 deaths2 a year are attributed to non-fire-related, unintentional CO poisoning cases in the U.S. However, large registry trials show the prevalence of CO poisoning is far greater with approximately 50,000 ED visits per year.3 Because the symptoms of CO poisoning are nonspecific—ranging from mild headache, nausea, confusion, and dizziness to end-organ injury, such as myocardial infarction, stroke, and death—diagnosis is difficult and has historically relied on clinical suspicion and confirmation by invasive blood gas analysis. Unfortunately, it has been estimated that up to half of U.S. hospitals do not have invasive COHb testing ability—increasing the potential that many victims of CO poisoning could be overlooked and misdiagnosed.4 Masimo SpCO allows immediate and noninvasive assessment of toxic CO levels—enabling early detection of CO poisoning that facilitates prompt, potentially life-saving treatment.
SpCO is part of the Masimo rainbow®SET platform—the first-and-only technology to noninvasively and continuously measure total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO), methemoglobin (SpMet), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the 'gold-standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR). Available in handheld (Masimo Rad-57™) and bedside devices (Masimo Radical-7™, Masimo Rad-87™), SpCO provides clinicians and emergency first responders with a way to quickly, noninvasively evaluate and address the significant health and safety risks associated with CO poisoning—helping to save and sustain lives, minimize suffering, and enhance safety. The new, enhanced versions of the Masimo rainbow Reusable Direct Connect SpCO and DCI®SpCO sensors (previous part numbers 2201, 2407, 2202, 2069, 2640, 2070, 2696, 2697) will now be identified as ID code H. Customers who would like to purchase the enhanced ID code H sensors should call (800) 257-3810 in the U.S., +41 32 720 1111 internationally, or their local sales representative.
1 Centers for Disease Control and Prevention. "Nonfatal, unintentional, non–fire-related carbon monoxide exposures—United States, 2004-2006." MMWR Morb Mortal Wkly Rep. 2008;57:896-899.
2 Centers for Disease Control and Prevention. "Carbon monoxide–related deaths—United States, 1999-2004." MMWR Morb Mortal Wkly Rep. 2007;56:1309-1312.
3 Keles A, Demircan A, Kurtoglu G. "Carbon monoxide poisoning: how many patients do we miss?" Eur J Emerg Med. 2008;15:154-157.
4 Hampson NB, Scott KL, Zmaeff JL. "Carboxyhemoglobin measurement by hospitals: implications for the diagnosis of carbon monoxide poisoning." J Emerg Med. 2006;31:13-16.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care… by Taking Non-invasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SpCO will provide an accurate and effective method of screening for CO poisoning in large numbers of at-risk populations, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at the 2011 Bank of America Merrill Lynch Health Care Conference
IRVINE, Calif., April 27, 2011 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the Bank of America Merrill Lynch Health Care Conference at the Encore at Wynn in Las Vegas on Wednesday, May 11, 2011, at 10 a.m. Pacific Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations,
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered


New Published Study Finds Masimo Noninvasive SpCO Effective for Screening Emergency Department Patients for Carbon Monoxide Poisoning
Irvine, California – April 21, 2011 – Masimo (NASDAQ: MASI) announced today that a new study published in this month's issue of the peer-reviewed journal, Annals of Emergency Medicine, demonstrates that noninvasive Masimo carboxyhemoglobin (SpCO®) measurements provide an "effective means for screening at-risk populations for CO poisoning" with "acceptable bias and precision" compared to invasive blood gas analysis. The prospective diagnostic accuracy study is more than ten times larger than any other published SpCO accuracy study to date and provides a strong rationale for clinical use of SpCO in the evaluation of emergency department (ED) patients.1
Carbon monoxide (CO) poisoning is a major cause of morbidity and mortality. At least 20,000 known exposures2 and 439 deaths3 a year are attributed to non-fire-related, unintentional CO poisoning cases in the U.S. However, large registry trials show the prevalence of CO poisoning is far greater with approximately 50,000 ED visits per year.4 Because the symptoms of CO poisoning are nonspecific—ranging from mild headache, nausea, confusion, and dizziness to end-organ injury, such as myocardial infarction, stroke, and death—diagnosis is difficult and has historically relied on clinical suspicion and confirmation by measurement of carboxyhemoglobin (COHb) via invasive blood gas analysis. Unfortunately, it has been estimated that up to half of U.S. hospitals do not have invasive COHb testing ability—increasing the potential that many victims of CO poisoning could be overlooked and misdiagnosed.5
The study, conducted over a year-long period in the Department of Emergency Medicine at one of the largest hospitals in Europe, the Vienna General Hospital (AKH Vienna), compared the accuracy of SpCO measured noninvasively using the Masimo Radical-7 with COHb measurements obtained via invasive blood gas analysis in 1,578 ED patients. Results showed a bias of 2.99% COHb (1.50% for smokers, 4.33% for nonsmokers) and a precision of 3.27% COHb (2.90% for smokers, 2.98% for nonsmokers) between SpCO and lab values—demonstrating "acceptable bias and precision" for the "detection of high concentrations of COHb, found in acute CO poisoning." Values ranged from 0-50% for SpCO and 0-39.3% for COHb with limits of agreement from -3.55% to 9.53% COHb (-4.30% to 7.30% for smokers, -1.63% to 10.29% for nonsmokers). A total of 17 patients (9 smokers, 8 nonsmokers) with a mean COHb of 14.1% received a final diagnosis of CO poisoning. Using a cut-off SpCO value of 6.6%, the researchers found a 94% sensitivity (ability to detect CO poisoning) and 77% specificity (ability to identify a lack of CO poisoning) and stated that the method "appears to be a reasonable upper limit of normal cutoff value for a screening test in the ED setting."
Researchers also found that several factors influence SpCO measurement accuracy as compared to COHb, including smoking, SpCO level, interval between measurements, and age. Because cigarettes increase CO levels in the blood, users were cautioned to pay attention to "the number of cigarettes smoked." Importantly, because CO in the blood decreases with time, researchers cited the "half-life of CO" and "time between multiwave pulse oximetry and blood gas analysis" as other possible "influencing factors on the deviation between SpCO and COHb." The study also shows that time between measurement was still a factor within the 60-minute cut-off established by the researchers—underscoring the importance of simultaneous comparisons between SpCO and invasive blood gas measurements due to the half-life of CO.
Study authors concluded that SpCO measurement via multiwave pulse oximetry has "an acceptable bias and precision" and "keeping influencing factors in mind, it can therefore be used to screen large numbers of patients for latent CO poisoning." They also noted the "potential for different measurement results from correct and incorrect (sensor) placement," an encouragement for users to closely follow the directions for use. The current study follows the results by researchers in the ED of a Rhode Island Hospital, who first demonstrated that large scale population screening of ED patients using SpCO could identify patients with unsuspected CO poisoning.6
1 Roth D, Herkner H, Schreiber W, Hubmann N, Gamper G, Laggner A, Havel C. "Accuracy of Noninvasive Multiwave Pulse Oximetry Compare with Carboxyhemoglobin From Blood Gas Analysis in Unselected Emergency Department Patients." Annals of Emergency Medicine Published April 2011 online ahead of print http://www.annemergmed.com/article/S0196-0644(10)01934-7/abstract.
2 Centers for Disease Control and Prevention. "Nonfatal, unintentional, non–fire-related carbon monoxide exposures—United States, 2004-2006." MMWR Morb Mortal Wkly Rep. 2008;57:896-899.
3 Centers for Disease Control and Prevention. "Carbon monoxide–related deaths—United States, 1999-2004." MMWR Morb Mortal Wkly Rep. 2007;56:1309-1312.
4 Keles A, Demircan A, Kurtoglu G. "Carbon monoxide poisoning: how many patients do we miss?" Eur J Emerg Med. 2008;15:154-157.
5 Hampson NB, Scott KL, Zmaeff JL. "Carboxyhemoglobin measurement by hospitals: implications for the diagnosis of carbon monoxide poisoning." J Emerg Med. 2006;31:13-16.
6 Suner S, Partridge R, Sucov A, et al. "Noninvasive Pulse CO-Oximetry Screening in the Emergency Department Identifies Occult Carbon Monoxide Toxicity." J. Emerg Med. 2008; 34(4): 441-450.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SpCO will provide an accurate and effective method of screening for CO poisoning in large numbers of at-risk populations, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Report First Quarter 2011 Financial Results after Market Close on May 3, 2011
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., April 19, 2011 -- Masimo (NASDAQ: MASI) announced today that it will release first quarter financial results for the period ended April 2, 2011, after the market closes on May 3, 2011.
A conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 58223656. After the live webcast, the call will be available on Masimo's website through June 3, 2011. In addition, a telephonic replay of the call will be available through May 17, 2011. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 58223656.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations,
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


New Published Study Finds Masimo SET® Delivers More Reliable Measurements Significantly Faster Than Other Pulse Oximetry Technologies
Use of Masimo SET Enables Better Adjustments During Critical Neonatal Resuscitation Situations, Aiding in the Prevention of Damage Caused by Unnecessary Exposure to High or Low Oxygen
Irvine, California – April 11, 2011 – Masimo (NASDAQ: MASI) announced today that a new study published in this month's issue of the European peer-reviewed journal Acta Paediatrica demonstrates that pulse oximetry makes a critical difference in neonatal resuscitation both in terms of reliability and speed of measurements. In comparing the measurement response times of three different pulse oximetry technologies, researchers found that the Masimo Radical-7™ pulse oximeter with Masimo SET®Measure-Through Motion and Low Perfusion technology displayed reliable oxygen saturation (SpO2) measurements three to four times faster than competing pulse oximeters.1
Despite numerous advances to improve newborn care, oxygen is still used liberally during newborn resuscitation—unnecessarily exposing many newborns to potentially damaging hyperoxia. Although pulse oximetry is an important clinical tool for evaluating a patient's oxygenation status and guiding resuscitation, measurement failure rates due to motion artifact and low perfusion can be high—leading to inaccurate readings, failure to report readings, or freezing of displayed values. As a result, the time to obtain a reliable oxygen saturation reading during newborn resuscitation in the delivery room and during NICU care is a critically important consideration when choosing a pulse oximetry technology. This is the first prospective observational study to compare pulse oximetry technology performance in detail during newborn resuscitation under unstable critical conditions.
The study was conducted at two health care centers in Barranquilla, Colombia (Clinica del Mar and Medicina Alta Complejidad S.A.) on 32 newborns (median gestational age of 32 weeks) receiving resuscitation as standard of care either in the delivery room or in the Neonatal Intensive Care Unit (NICU). Using the Masimo LNOP®sensor with the Radical-7, the E630 sensor with the Ohmeda Biox 3700, and the OxiMax Max-N sensor for the Nellcor N395, the time to a reliable SpO2 measurement was recorded post-ductally (one sensor placed on each foot) once an adequate pulse rate signal was displayed. Results showed that "the pulse oximeter with Masimo SET provided the fastest response time." In the first comparison of 17 infants, the median response time to obtain a reliable measurement for the Masimo Radical-7 was 20.2±6 (with a range of 18-26 seconds) versus 74.2±12 (with a range of 38-98 seconds) for the Ohmeda Biox 3700. In the second comparison of 15 infants, the median response time for the Masimo Radical-7 was 20.9±4 (with a range of 19-28 seconds) versus 67.3±21 (with a range of 40-90 seconds) for the Nellcor N-395.
Finding that "there are significant differences in the response of pulse oximeters during neonatal resuscitation," researchers concluded that "the speed and reliability of the Masimo SET technology can be of help for clinicians to more accurately adjust the fraction of inspired oxygen during newborn resuscitations, thus preventing or minimizing damage secondary to unnecessary exposure of oxygen and hyperoxemia and to wide fluctuations in oxygen levels."
The 'gold standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® has been shown in over 100 independent clinical studies to provide the most accurate and trustworthy measurements—even under the most challenging clinical conditions, including patient motion and low perfusion.
1 Baquero H, Alviz R, Castillo A, Neira F, Sola A. "Avoiding Hyperoxemia During Neonatal Resuscitation: Time To Response Of Different SpO2 Monitors." Acta Paed April 2011 Vol. 100, Issue 4, pp 515-518. For further reading, visit: http://onlinelibrary.wiley.com/doi/10.1111/j.1651-2227.2010.02097.x/abstract
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SET delivers more reliable measurements significantly faster than other pulse oximetry technologies and the use of Masimo SET enables better adjustments during neonatal resuscitation situations, which may aiding in the prevention of damage caused by unnecessary exposure to high or low oxygen, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Newtech, Inc., a Leading Chinese EMS Patient Monitoring Company, Partners with Masimo to Incorporate rainbow® SET Noninvasive Measurement Capabilities into New Patient Monitors
Shenzhen, China and Irvine, California – March 28, 2011 – Newtech, Inc., and Masimo Corporation (NASDAQ: MASI) jointly announced today a new technology integration strategy that will make the Masimo rainbow SET Pulse CO-Oximetry™ technology platform a fundamental offering in Newtech's emergency medical services (EMS) and multiparameter product line-up—providing clinicians with a host of innovative new noninvasive, continuous measurements and patient monitoring capabilities for the immediate detection of potentially life-threatening conditions.
"By incorporating the Masimo rainbow SET technology into our products, we will be able to offer the EMS market in China the most advanced technologies in monitoring EMS patients," said Ganbing Wang, President of Newtech, Inc. "We see huge potential within China's EMS market over the next five years because of strong government investment to upgrade the EMS system and, being an innovative medical device company, we are pleased to partner with Masimo in providing the best technology for our customers to meet not only their current demands, but their future demands as well."
Masimo rainbow SET noninvasive and continuous measurements—like total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and rainbow Acoustic Monitoring™ (RAM™), in addition to the "gold-standard" Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR)—provide detailed physiological data that helps clinicians to more rapidly assess patients and detect adverse conditions earlier. Immediate availability of such detailed clinical intelligence and measurement data enables clinicians to proactively detect and treat the early signs of hemodynamic instability, potentially life-threatening conditions and internal bleeding sooner rather than reacting to late indicators and their deleterious effects.
Newtech's utilization of broadband wireless technologies allows seamless transmission of patient-critical vital sign information over China's Emergency Medical 3G and 4G networks and the internet. This real-time, live streaming of video, audio and vital signs data provides caregivers with the ability to immediately assess and treat their patients, at the point of care or during ambulance transport. Information is transmitted by the multiparameter handheld (NT1D) or NT3C and NT5 ambulance mounted patient monitors, to a base station, which may be either a remote centralized monitoring system or hospital Emergency Department, where caregivers are alerted in advance and may prepare treatment regimens.
Pre-hospital/EMS early interventions have been shown to dramatically reduce "call to needle" or "door to needle" treatment times, especially in rural areas, resulting in lower mortality rates, improved patient outcomes and survival rates. Incorporating Masimo rainbow SET technology expands the clinician's ability to assess and treat the pre-hospital/EMS patient to now include noninvasive monitoring of critical blood constituents such as hemoglobin (SpHb), Pleth Variability Index (PVI), and carboxyhemoglobin (SpCO), so that a caregiver may evaluate and treat for blood loss, anemia, and even suspected carbon monoxide poisoning before the patient has reached the hospital.
Masimo President of Worldwide OEM Business & Business Development, Rick Fishel, stated, "Masimo's mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. Newtech's innovative approach to real-time monitoring of patients—in remote/rural settings, during ambulance transport, at the point of care, or wherever the care setting may be—represents a shared vision to bring technology to the patient rather than the patient to the technology, so that care may begin as early as possible. We are very pleased that Masimo's unique rainbow Pulse CO-Oximetry platform is a key component in Newtech's new patient monitors."
About Newtech, Inc.
Founded in 1998, Newtech, Inc. is one of the fastest growing medical device manufacturers in China. The company designs, manufactures and markets patient monitoring systems with a current emphasis in EMS monitoring systems. Located in Shenzhen, China, the company was certified by the city government as a "Shenzhen High-tech Enterprise" in 2001. It's quality system was certified to ISO13485 by TüV PS in 2002. The company's products are all SFDA cleared for China market, with many also CE marked or FDA cleared for worldwide distribution. Additional information about Shenzhen Newtech. Inc. and its products is available on the company's website: www.sznewtech.com.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Non-invasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the timing and market availability of Shenzhen Newtech products with Masimo rainbow SET technology, risks related to our assumptions that the integration of Masimo rainbow SET provides advanced noninvasive and continuous measurements and patient monitoring capabilities that have the potential to improve clinical decision-making, outcomes, and patient safety, risks related to our belief that Masimo rainbow SET measurements will enable clinicians to detect potentially life-threatening conditions earlier in all patients and clinical settings, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
For More Information Contact:
Rachel Cheng
NEWTECH, Inc.
650.588.3980
Rachel@solaris-newtech.com
Dana Banks
Masimo Corporation
949.297.7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Non-invasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation. All trademarks and registered trademarks are the sole property of their respective companies.


New Studies Show Clinical Advantages of Masimo Noninvasive Hemoglobin, PVI, and Perfusion Index in Critically-ill Patients
Presented at the International Symposium of Intensive Care and Emergency Medicine (ISICEM) in Belgium
Irvine, California – March 24, 2011 – Masimo (NASDAQ: MASI) announced today that three new studies presented at the International Symposium of Intensive Care and Emergency Medicine (ISICEM) Annual Meeting in Brussels, Belgium,demonstrate the accuracy, reliability, and clinical impact of Masimo noninvasive and continuous measurements—noninvasive hemoglobin (SpHb®), Pleth Variability Index (PVI®), and Perfusion Index (PI)—in Intensive Care Unit (ICU) patients.
"Accuracy of a Continuous Noninvasive Hemoglobin Monitor in Intensive Care Unit"1
Researchers at the Centre Hospitalier Universitaire in Poitiers, France compared the absolute and trend accuracy of Masimo noninvasive hemoglobin (SpHb) to other invasive methods of hemoglobin assessment—including a point of care device (HemoCue301), a satellite lab CO-Oximeter (Siemens RapidPoint 450), and a 'gold-standard' laboratory hematology analyzer (Sysmex XT-2000i)—in 62 critically-ill ICU patients requiring 471 invasive blood samples. Compared to the gold-standard Sysmex hematology analyzer, results showed a bias and limits of agreement for Masimo noninvasive SpHb of 0.0±1.0g/dL. Compared to the gold-standard hematology analyzer, the invasive Hemocue 301 and Rapidpoint 450 had a bias and limits of agreement of 0.3±1.3 g/dL and 0.9±0.6 g/dL, respectively. Citing "absolute and trending accuracy similar to widely used invasive methods," researchers concluded that Masimo SpHb has "the additional advantages of providing continuous measurements, noninvasively, which may facilitate hemoglobin monitoring in the ICU."
"Pleth Variability Index Predicts Fluid Responsiveness in Critically-ill Patients"2
To evaluate whether Masimo PVI, a noninvasive and continuous tool, can help clinicians predict fluid responsiveness in mechanically-ventilated patients with circulatory insufficiency, researchers at the Centre Hospitalier Universitaire in Poitiers, France measured PVI in 40 patients before and after planned volume expansion. Fluid challenge consisted of either 500mL of 130/0.4 hydroxyethyl-starch (fluid) if respiratory variations in arterial pulse pressure > 13%, or with passive leg raising (PLR) otherwise. Results showed 21 patients (19 fluid, 2 PLR) were responders—defined as an increase in cardiac output of >15%. A PVI threshold value of 17% allowed discrimination between responders and non-responders with a sensitivity of 95% (95% confidence interval, 74-100%) and a specificity of 91% (95% confidence interval, 70-99%), leading researchers to conclude that "PVI can predict fluid responsiveness noninvasively in ICU patients under mechanical ventilation." Additionally, PVI at baseline correlated (r=0.72, p<.0001) with changes in cardiac output induced by fluid challenge—suggesting the higher PVI at baseline, the higher the percentage change in cardiac output after volume expansion.
"Perfusion Index as a Predictor for Central Hypovolemia in Humans"3
In this study—the first to evaluate the ability of Masimo PI to detect peripheral vasoconstriction due to neurohumoral response in central hypovolemia-induced by lower body negative pressure (LBNP)—researchers from Erasmus MC University Medical Centre in Rotterdam, Netherlands measured PI in 24 healthy volunteers during LBNP testing. LBNP consisted of 5 min. baseline measurements in the supine position followed by stepwise increases of negative pressure from 0 to -20, -40, -60, -80, and o mmHg. Results showed that PI decreased significantly by 40% (P=0.03) during first -20mmHg and stayed in this range during the remainder of the experiment while stroke volume (SV) decreased by 20% and heart rate (HR) increased by 15% at -40mmHg and were proportional to the level of negative pressure in the chamber. Researchers concluded that PI is a "sensitive indicator of acute hemodynamic responses to LBNP-induced central hypovolemia" and could "detect hypovolemia earlier than 20% decrease in stroke volume."
SpHb, PVI, and PI are available as part of Masimo rainbow®SET platform—the first-and-only technology to noninvasively and continuously measure total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), perfusion index (PI), and acoustic respiration rate (RRa™), in addition to the 'gold standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), and pulse rate (PR).
1 Frasca D, Dahyot-Fizelier C, Karen C. Levrat Q, Soumagne-Vialle N, Boisson M, Debaene B, Mimoz O. "Accuracy of a Continuous Noninvasive Hemoglobin Monitor in Intensive Care Unit." CHU Poitiers, Anesthésie-Réanimation INSERM ERI 23, POITIERS, France. ISICEM Presentation A91.
2 Nanadoumgar H, Loupec TL, Frasca DF, Petitpas FP, Laksiri LL, Baudouin DB, Mimoz OM. "Pleth Variability Index Predicts Fluid Responsiveness in Critically-ill Patients."CHU POITIERS, Reanimation Chirurgicale, Poitiers Cedex, France. ISICEM Presentation A532.
3 Lima A, Van Genderen M, Klijn E, Bartels S, Van Bommel J, Bakker J."Perfusion Index as a Predictor for Central Hypovolemia In Humans." Erasmus MC University Medical Centre Rotterdam, Intensive Care, Rotterdam, Netherlands. ISICEM Presentation A422.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our assumptions that Masimo SpHb accurately tracks and trends Hb changes in all patients, Masimo PVI is accurately predicts fluid responsiveness in all mechanically-ventilated, and Masimo PI is capable of detecting acute LBNP-induced central hypovolemia earlier than stroke volume in all ICU patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at the Roth Growth Stock Conference
IRVINE, Calif., March 3, 2011 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the Roth 23nd Annual Growth Stock Conference at the Ritz-Carlton in Laguna Niguel, Calif. on Tuesday, March 15, 2011, at 9:00 a.m. P.T. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations,
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Royal Fornia Medical Equipment Co., Ltd. Signs Technology Licensing Agreement to Integrate Masimo rainbow SET Pulse CO-Oximetry into New Patient Monitors
One of China's Largest Medical Manufacturers Establishes Pulse CO-Oximetry as the Blood Constituent Monitoring Platform of Choice for New Generation Multiparameter Monitors
Irvine, California & Guangdong Province, China – February 24, 2011 – Masimo (NASDAQ: MASI) and Royal Fornia Medical Equipment Co., Ltd. jointly announce a worldwide technology licensing agreement to integrate Masimo rainbow SET®Pulse CO-Oximetry™ technology into Royal Fornia's new generation of multiparameter patient monitors—enabling advanced noninvasive blood constituent measurements that help clinicians to detect and treat life-threatening conditions earlier. Having recently received State Food and Drug Administration (SFDA) approval for Masimo rainbow®SET parameters in China, Royal Fornia establishes itself as one of the first domestic Chinese medical device manufacturers to commercially incorporate Masimo's breakthrough noninvasive blood constituent measurement capabilities.
Masimo rainbow®SET noninvasive and continuous measurements—like total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to the 'gold-standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR)—provide detailed physiological data that helps clinicians to more rapidly assess patients and detect adverse conditions much earlier. With rainbow®SET measurements, the ability to noninvasively detect internal bleeding, carbon monoxide poisoning, methemoglobinemia, fluid responsiveness, low perfusion, desaturations, and abnormal pulse rates quickly, easily, and continuously can help save lives and improve patient outcomes. Immediate availability of such detailed clinical intelligence and measurement data enables clinicians to proactively detect and treat the early signs of hemodynamic instability, potentially life-threatening conditions, and bleeding sooner rather than reacting to late indicators and their deleterious effects.
According to Mr. Li Yang, Vice President of Royal Fornia Medical Equipment Co., Ltd, "Our corporate mission is to provide innovative medical devices with superior performance and value. The integration of Masimo rainbow®SET technology will allow us to design and manufacture state-of-the-art patient monitors that advance the delivery of healthcare. We expect that our combined product offering—Masimo's rainbow®SET measurements within our JCY-800-I and JCY-800-III multiparameter monitors—will provide clinicians with critical access to the latest cutting-edge technologies that allow them to more thoroughly assess a patient's physiological status and make better clinical and treatment decisions across the continuum of care."
Masimo President of Worldwide OEM Business and Corporate Development, Rick Fishel, stated, "Royal Fornia's 'first-to-market' commitment and strategy provides its customers with easy access to an upgradeable platform of blood constituent monitoring parameters that provide new clinical intelligence and detailed physiological data to improve patient safety and care while optimizing reimbursement potential—all consolidated in their new portable line of multiparameter patient monitors. Royal Fornia has a history of developing innovative technology solutions to address previously unmet clinical needs, and we are very pleased that Masimo's unique rainbow®Pulse CO-Oximetry platform is a key component in their new portable patient monitors. "
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
About Royal Fornia Medical Equipment Co., Ltd.
As a China-US joint venture, Royal Fornia Medical Equipment Co., Ltd. was founded in 2000 and is engaged in R&D, manufacturing and distributing of high-end medical electronic devices. Now, it has comprehensive sales network and well-developed after-sales service, which further facilitate and improve the promotion of its products. We are a group of professionals who follow the concept of "Human-oriented technology" and it contributes a lot to Royal Fornia's rapid growth. Under the guidance of our business philosophy "Health Care for Better Future," Royal Fornia will always pursue the advanced technologies and outstanding product quality, and will devote itself to the making of an excellent corporation in the high-tech industry. For more information visit: www.rfornia.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that Masimo rainbow SET Pulse CO-Oximetry provides detailed physiological data that helps clinicians to more rapidly assess all patients and detect adverse conditions much earlier; risks related to our belief that rainbow measurements can noninvasively detect internal bleeding, carbon monoxide poisoning, methemoglobinemia, fluid responsiveness, low perfusion, desaturations, and abnormal pulse rates quickly, easily, and continuously for all patients; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, Pulse CO-Oximetry, Pulse CO-Oximeter, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57, Rad-8, Rad-5, Pronto-7, Pronto, Patient SafetyNet, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Present at the Cowen and Company Health Care Conference
IRVINE, Calif., February 22, 2011 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the Cowen and Company Health Care Conference at The Marriott Copley Place in Boston on Tuesday, March 8, 2011, at 8:45 a.m. E.T. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations,
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation


Mater Children's Hospital in Australia Converts to Masimo rainbow SET for State-of-the-Art Noninvasive Patient Monitoring Capabilities
Queensland's Leading Pediatric Hospital Standardizes to Pulse CO-Oximetry—Offering Advanced Monitoring of Blood and Multiple Other Physiological Parameters Without Invasive Procedures
Irvine, California & Queensland, Australia – February 21, 2011 – Mater Children's HospitalandMasimo (NASDAQ: MASI) today jointly announce the conversion of Mater Children's Hospital to Masimo rainbow®SET Pulse CO-Oximetry™ technology for advanced noninvasive patient monitoring capabilities. The conversion equips the tertiary facility with the most technologically and clinically-advanced oximetry and noninvasive patient monitoring solutions available.
Masimo rainbow®SET Pulse CO-Oximetry is the first-and-only technology platform that allows hospitals to noninvasively measure and continuously monitor blood constituents and physiological parameters that previously required invasive procedures—including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the 'gold-standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR). The oximetry standard-of-care at leading hospitals worldwide, Masimo rainbow®SET provides immediate real-time results that enable clinicians to more rapidly assess patients and detect and treat adverse, potentially life-threatening conditions earlier.
"Converting to Masimo rainbow®SET provides our clinicians with superior patient monitoring capabilities and advanced clinical measurements thereby ensuring that our clinicians have access to quality clinical data," stated Malcolm Thatcher, Chief Information Officer for Mater Health Services. "The decision to standardize on Masimo's technology platform across our pediatric services was driven by the knowledge that, in harnessing the advanced noninvasive capabilities of Masimo rainbow®SET measurements, Mater is able to deliver an exceptional level of care and safety to our pediatric patients while recognizing the importance of the patient experience and supporting our organization's commitment to putting the patient first."
A major referral center for specialty pediatric healthcare services in Queensland, Mater Children's Hospital provides a range of inpatient, outpatient, and emergency care to over 167,000 children a year. The conversion ensures that all multiparameter patient monitors, stand-alone oximeters, and sensors feature the advanced noninvasive patient monitoring capabilities of Masimo rainbow®SET—allowing clinicians to easily and painlessly obtain and continuously track vital blood and physiological data for patients in real-time, anywhere in the hospital.
Mater Children's Hospital Pediatric Sleep Scientist, Gordon Williams, said the Sleep Unit relies on Masimo SET's ability to measure oxygen saturation during movement, which he says is "especially important in a pediatric facility where motion-artifact can be a major source of oximeter signal noise. Not only does Masimo's technology allow us to obtain more reliable saturation trend data in children being investigated in the sleep laboratory, but we are able to confidently use the Masimo Radical-7 Pulse CO-Oximeter as a screening and assessment tool on the wards as well. And in terms of future needs, Masimo's rainbow®SET Pulse CO-Oximetry platform positions us for growth well beyond how oximeters have traditionally been used to date."
Masimo Founder and CEO, Joe Kiani, stated, "Reducing the trauma of invasive tests and procedures for pediatric patients has long been a concern for every clinician and parent that cares for a sick child. Today, thanks to medical advancements enabled by Masimo rainbow®SET Pulse CO-Oximetry and the forward-thinking efforts of Mater Children's Hospital, pain-free hemoglobin testing and clinically reliable pulse oximetry is now a reality for pediatric patients in Queensland."
About Mater Children's Hospital
Mater Children's Hospital is a major tertiary pediatric referral centre with the busiest pediatric emergency department in Queensland. The hospital provides specialty healthcare services to more than 15,000 inpatient, 120,000 outpatient, and 32,000 emergency patients each year. To learn more about Mater, visit www.mater.org.au/Home/Hospitals/Mater-Children-s-Hospital.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the hospital-wide conversion ensures that all Mater Children's Hospital patients will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo rainbow SET provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient; risks related to our belief that SpHb detects low or falling hemoglobin levels that could be the result of internal bleeding; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Brooke Falvey
Mater Children's Hospital
(07) 3163 3495
brooke.falvey@mater.org.au
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, Pulse CO-Oximetry, Pulse CO-Oximeter, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57, Rad-8, Rad-5, Pronto-7, Pronto, Patient SafetyNet, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Reports Fourth Quarter and Full Year 2010 Financial Results; Provides 2011 Guidance
Q4 2010 Highlights (compared to Q4 2009):
- Total revenue rose 14.0% to $105.6 million
- Product revenue rose 16.6% to $93.8 million
- Masimo SET and Masimo rainbow SET unit shipments rose 37.5% to 41,800
- Masimo rainbow revenue rose 44.4% to $8.4 million
- GAAP EPS rose 13.0% to $0.26. Excluding one-time expenses, adjusted EPS rose 26.1% to $0.29.
Full Year 2010 Highlights (compared to 2009)
- Total revenue rose 16.1% to $405.4 million
- Product revenue rose 18.8% to $356.4 million
- Masimo SET and Masimo rainbow SET unit shipments rose 37.5% to 153,200
- Masimo rainbow revenue rose 69.0% to $32.9 million
- GAAP EPS rose 37.5% to $1.21. Excluding one-time items, adjusted EPS rose 17.1% to $1.03.
Irvine, California, February 15, 2011 – Masimo (NASDAQ: MASI) today announced its financial results for the fourth quarter and year ended January 1, 2011.
Masimo's total revenue for the fourth quarter rose 14.0% to $105.6 million, compared to $92.6 million for the fourth quarter of 2009. Masimo's fourth quarter product revenue rose 16.6% to $93.8 million, compared to $80.5 million for the fourth quarter of 2009. Revenue from Masimo rainbow products rose 44.4% to $8.4 million in the fourth quarter, compared to $5.8 million for the fourth quarter of 2009.
Net income for the fourth quarter was $16.1 million, or $0.26 per diluted share, including $0.02 per diluted share in one-time marketing and other related expenses that Masimo had previously planned and announced after receiving $30.8 million in proceeds in 2010 from an antitrust lawsuit against Covidien. Excluding these one-time marketing and other related expenses, adjusted net income for the fourth quarter was $17.5 million, or $0.29 per diluted share, compared to net income of $14.1 million, or $0.23 per diluted share, in the fourth quarter of 2009. Due to rounding, the impact of excluding $0.02 per diluted share in one-time marketing and other related expenses added $0.03 per diluted share to the adjusted earnings per share calculation in the fourth quarter of 2010.
For 2010, Masimo's total revenue rose 16.1% to $405.4 million, compared to $349.1 million for 2009. The company's product revenue rose 18.8% in 2010 to $356.4 million, compared to $300.1 million in 2009. Revenue from Masimo rainbow products rose 69.0% to $32.9 million in 2010, compared to $19.5 million in 2009.
Masimo's net income for 2010 was $73.5 million, or $1.21 per diluted share, including $0.18 per diluted share from the net of a one-time gain related to the $30.8 million in antitrust proceeds from Covidien and offset by $14.7 million in marketing and other related expenses that Masimo had previously planned and announced after receiving the proceeds. Excluding these one-time items, adjusted net income for 2010 was $62.5 million, or $1.03 per diluted share, compared to net income of $53.2 million, or $0.88 per diluted share, in 2009.
During the fourth quarter, the company shipped approximately 41,800 Masimo SET pulse oximetry and Masimo rainbow SET Pulse CO-Oximetry units, excluding handheld units, up 37.5% compared to approximately 30,400 in the same prior year period. For 2010, Masimo shipped approximately 153,200 Masimo SET pulse oximetry and Masimo rainbow SET Pulse CO-Oximetry units, excluding handheld units, up 37.5% compared to approximately 111,400 in 2009. Masimo estimates its worldwide installed base as of January 1, 2011 to be 855,000 units, up 18.0% from 724,000 units as of January 2, 2010.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "Our strong fourth quarter and fiscal 2010 performance was driven by increasing global demand for our breakthrough Masimo SET and Masimo rainbow SET technologies, allowing us to advance our mission to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications."
As of January 1, 2011, cash, cash equivalents and short-term investments totaled $88.3 million, compared to $189.0 million as of January 2, 2010. The decline was due primarily to special dividend payments of $117.5 million and $44.5 million, made on March 31, 2010 and December 21, 2010, respectively. Total 2010 dividend payments of $162.0 million were partially offset by the net proceeds from the Covidien antitrust lawsuit and operating cash flow during the year.
2011 Financial Guidance
Masimo expects fiscal 2011 total revenue to be between $446 million and $463 million, including product revenue in the range of $415 million to $430 million, and royalty revenue in the range of $31 million and $33 million. Included within these product revenue ranges are 2011 rainbow revenue expectations of $40 million to $50 million. As a result, the company expects fiscal 2011 GAAP earnings per share to be between $1.17 and $1.25. Each of the components of Masimo's guidance set forth above is an estimate only and actual performance could differ.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 679-9937 for international callers. The reservation code for both dial-in numbers is 38781834. After the live webcast, the call will be available on Masimo's website through March 15, 2011. In addition, a telephonic replay of the call will be available through March 1, 2011. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 38781834.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can also be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies; and expectations for total revenue, royalty revenue and product revenue, including rainbow revenue, and GAAP earnings per share for the full fiscal year 2011. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations,
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.





Mafraq Hospital Becomes First in United Arab Emirates to Deploy Masimo rainbow SET Pulse CO-Oximetry for State-of-the-Art Noninvasive Patient Monitoring
Largest Hospital in the UAE Converts to Pulse CO-Oximetry Technology—Offering Advanced Monitoring of Blood Constituents and Multiple Physiological Parameters Without Invasive Procedures
Irvine, California and Dubai, UAE – February 10, 2011 – Mafraq Hospital andMasimo (NASDAQ: MASI) jointly announced the hospital-wide conversion of Mafraq Hospital to Masimo rainbow®SET Pulse CO-Oximetry™ technology for advanced noninvasive patient monitoring capabilities. The conversion makes Mafraq Hospital the first hospital in the UAE to feature Masimo's technologically and clinically-advanced oximetry and noninvasive patient monitoring solutions.
The Masimo rainbow SET Pulse CO-Oximetry technology platform allows hospitals to noninvasively measure and continuously monitor multiple blood constituents and physiological parameters that previously required invasive procedures—including: total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the 'gold-standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR). The oximetry standard-of-care at leading hospitals worldwide, Masimo rainbow SET provides immediate real-time results that enable clinicians to more rapidly assess patients and detect and treat adverse, potentially life-threatening conditions earlier.
"Mafraq Hospital takes great pride in the skills and experience of its physicians and the fact that we have some of the world's most advanced equipment available, so that we can provide the best medical care and treatment services in one of the fastest growing areas in the Emirate of Abu Dhabi," stated John Nickens, CEO at Mafraq Hospital. "We saw tremendous value in the superior performance and futuristic noninvasive capabilities of Masimo rainbow SET Pulse CO-Oximetry and wanted to extend its positive clinical impact and patient care benefits to all of our patients throughout the hospital."
Mafraq Hospital, managed by Bumrungrad International and owned and operated by the Abu Dhabi Health Services Company PJSC (SEHA), which is responsible for all the curative activities of public hospitals and clinics in Abu Dhabi, is a major referral hospital and comprehensive tertiary healthcare provider in the UAE for 510,000 patients each year. The conversion ensures that all multiparameter patient monitors, stand-alone oximeters, and sensors throughout the hospital will feature the advanced noninvasive patient monitoring capabilities of Masimo rainbow SET—allowing Mafraq Hospital clinicians to easily and painlessly obtain and continuously track vital blood and physiological data for patients in real-time. Mafraq Hospital initially launched the new technology platform hospital-wide with Masimo SET pulse oximetry measurements (SpO2, PI, and PR), enabling the hospital to quickly and easily upgrade to Masimo rainbow Pulse CO-Oximetry measurements (SpHb, SpOC, PVI, SpCO, SpMet) as needed.
According to Mafraq Hospital Chief Nursing Officer, Gail Smith, "This conversion provides an exciting new medical first—noninvasive and continuous hemoglobin (SpHb), which allows our clinicians to perform trauma-free hemoglobin blood tests and get immediate results. And, as the only hospital in the UAE equipped with SpHb, we not only have the ability to quickly measure hemoglobin levels without removing a drop of blood from the patient, but we can now continuously track hemoglobin levels in real-time without having to perform repeated, painful blood draws and time-consuming lab analyses."
Masimo Founder and CEO, Joe Kiani, stated, "We are happy to be partnering with Mafraq Hospital as they lead the UAE in advancing the care and safety of patients. Mafraq Hospital's adoption of Masimo rainbow SET now makes it possible for hospital clinicians to noninvasively monitor and continuously track key indicators of a patient's relative physiological status—including hemoglobin and other blood constituents as well as fluid volume and respiration rate—to immediately and painlessly identify abnormalities and detect potentially life-threatening conditions much earlier."
The CEO of Emitac Healthcare Solutions (EHS), Masimo's distributor in the UAE, Mr. Raghavan Srinivasan considers it a matter of pride to partner with Masimo in deploying this solution to Mafraq Hospital, stating that: "This is a progressive step by the hospital to adopt innovative means to providing quality healthcare in the country."
About Mafraq Hospital
Mafraq Hospital was established in 1983 and is one of the largest tertiary referral treatment hospitals in the United Arab Emirates, with 451 licensed beds. Its specialist services include medicine, obstetrics, pediatrics, as well as surgical and critical care services. Mafraq Hospital operates the largest burn unit in the country and is a Center of Excellence for ENT and Thoracic surgery. The hospital also operates two primary healthcare clinics at Bani Yas and Al Nahda. Mafraq Hospital is part of the SEHA Health System and is owned and operated by Abu Dhabi Health Services Company PJSC (SEHA) which is responsible for the curative activities of the public hospitals and clinics of the Emirate of Abu Dhabi. It is managed by Bumrungrad International (BIL), one of the most noted hospital management companies in Asia. Mafraq Hospital physicians are amongst the best in their specialist fields with international qualifications and world class expertise. The medical staff is supported by a capable and professional nursing and paramedical staff who are well trained and motivated to provide quality healthcare services. Emiratization is extremely important to Mafraq Hospital and many of the hospital's highly skilled physicians are UAE Nationals. The hospital also believes in customer service excellence and in providing quality healthcare with integrity and service excellence in a caring environment.
About the SEHA Health System and the Abu Dhabi Health Services Company PJSC (SEHA)
SEHA is health in Arabic. The Abu Dhabi Health Services Company PJSC – whose marketing identity is SEHA – is an independent, public joint stock company created to develop the curative activities of the public healthcare system in Abu Dhabi. The company owns and operates all the public hospitals and clinics of the Emirate of Abu Dhabi which together make up the SEHA Health System. SEHA is committed to providing high quality, cost effective healthcare in a socially responsible way on par with international standards measured through accessibility, affordability, choice and satisfaction. SEHA has partnered with internationally recognized hospital managers to achieve these goals. These include Johns Hopkins Medicine International, Cleveland Clinic Foundation, VAMED, Vienna Medical University and Bumrungrad International. SEHA owns and operates 12 hospitals with 2,644 beds, 62 ambulatory care, family care and urgent care centers and 2 blood banks. SEHA is one of the largest integrated healthcare providers in the Middle East with 16,500 doctors, nurses, ancillary care and administrative personnel in its employ. For more information, visit www.seha.ae.
About Masimo
Masimo (NASDAQ: MASI) develops innovative monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®Pulse CO-Oximetry™, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the hospital-wide conversion ensures that all Mafraq patients will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo rainbow SET provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient; risks related to our belief that SpHb detects low or falling hemoglobin levels that could be the result of internal bleeding; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Amanda Banham
Mafraq Hospital
+971 2 5012443
abanham@mafraqhospital.ae
Dana Banks
Masimo Corporation
+1(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, Pulse CO-Oximetry, Pulse CO-Oximeter, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57, Rad-8, Rad-5, Pronto-7, Pronto, Patient SafetyNet, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Welch Allyn Establishes Strategic Partnership to Incorporate Masimo rainbow® SET Noninvasive Measurement Capabilities in Next-Generation Patient Monitors
Company will integrate Masimo's advanced Pulse CO-Oximetry™ technology into its new Connex®Vital Signs Monitor and future product releases
Skaneateles Falls, New York and Irvine, California – February 8, 2011 – Welch Allyn and Masimo (NASDAQ: MASI) jointly announced today a new technology integration strategy that will make the Masimo rainbow®SET Pulse CO-Oximetry™ technology platform a strategic and fundamental offering in Welch Allyn's new product line-up—providing clinicians with a host of innovative new noninvasive, continuous measurements and patient monitoring capabilities. In addition, the unique upgradeability inherent within the rainbow SET technology platform, coupled with the flexibility and configurability of next-generation Welch Allyn patient monitors, will allow clinicians the option to remotely add Masimo's noninvasive and continuous measurements as optional software upgrades as the measurements become commercially available for use with the Welch Allyn patient monitor.
"Welch Allyn is excited to provide our customers with the option of enabling the Masimo parameters within our patient monitoring devices in a manner that considers their evolving needs over time," said Welch Allyn Executive Vice President and Chief Global Business Officer, Steve Meyer. "Masimo technology supports our platform concept of modularity and upgradeability while allowing customers to improve patient care through early, noninvasive continuous and spot check monitoring of key hemodynamic parameters."
Masimo rainbow SET noninvasive and continuous measurements—like total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and rainbow Acoustic Monitoring™ (RAM), in addition to the "gold-standard" Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR)—provide detailed physiological data that helps clinicians to more rapidly assess patients and detect adverse conditions earlier. Pending regulatory clearance, Welch Allyn will announce the commercial availability of these rainbow SET parameters as they become legally available for use with its monitors.
Immediate availability of such detailed clinical intelligence and measurement data enables clinicians to proactively detect and treat the early signs of hemodynamic instability, potentially life-threatening conditions and internal bleeding sooner rather than reacting to late indicators and their deleterious effects.
The new Welch Allyn Connex®Vital Signs Monitor (CVSM) is the first Welch Allyn monitor to integrate the Masimo rainbow SET technology. The CVSM, which launched in August 2010, incorporates Masimo's SpO2-enabled measurements and is capable of being field upgraded to add rainbow SET-based measurements as each parameter becomes commercially available for use with the CVSM.
The CVSM is a full-color, touch screen device that acts as three devices in one—providing comprehensive patient documentation on a single display. This documentation includes automatic measurements such as heart rate, blood pressure, temperature and pulse oximetry; manual parameters such as respiration, height, weight and pain level; and modifiers such as body position, O2 therapy details and others. The device also gives the clinician the ability to control alarms, patient data and monitoring in a customized manner for each patient, and document the data right on the device—eliminating the need to locate a PC and transcribe it later.
Welch Allyn will offer customers the ability to remotely upgrade the device's software to add additional Masimo rainbow SET capabilities via the Web using the company's innovative PartnerConnect™ Remote Services solution. PartnerConnect is a feature of the Welch Allyn Partners in Care ServicesSM program that enables remote diagnostics of devices and systems. Software installations, updates, and upgrades can all be delivered and performed remotely. PartnerConnect also enables preemptive service capabilities on the Welch Allyn platform, allowing the Partners in Care Technical Support Center to remotely view devices and system configurations in real time via the Internet.
Masimo President of Worldwide OEM Business, Business Development, and Masimo Americas, Rick Fishel, stated, "Integrating industry-leading noninvasive and continuous monitoring technologies that offer point-of-care, cost-effective measurements and documentation is a growing trend in both continuous and spot-check patient monitors. The Welch Allyn Connex Vital Signs Monitor represents an innovative monitoring platform in many ways, including its point-of-care configurability, upgradeability, wireless EHR connectivity, and next-generation patient monitoring capabilities—all of which help clinicians meet the patient care challenges and clinical demands of today and tomorrow. We are proud of the positive impact our medical technology innovations have on patient care, safety, and clinical outcomes, and we are equally proud to be a key part of Welch Allyn's forward-looking patient monitoring technology strategy."
About Welch Allyn
Founded in 1915 and headquartered in Skaneateles Falls, NY (USA), Welch Allyn is a leading global provider of medical diagnostic equipment and a complete range of digital and connected solutions. With 2,600 employees working in 26 different countries, Welch Allyn specializes in helping doctors, nurses, and other frontline practitioners across the globe provide the best patient care by developing innovative products, breakthrough technologies, and cutting- edge solutions that help them see more patients, detect more conditions, and improve more lives. More information about Welch Allyn and its complete line of connected products and solutions may be found at www.welchallyn.com
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the timing and market availability of Welch Allyn products with Masimo rainbow SET technology, risks related to our assumptions that the integration of Masimo rainbow SET provides advanced noninvasive and continuous measurements and patient monitoring capabilities that have the potential to improve clinical decision-making, outcomes, and patient safety, risks related to our belief that Masimo rainbow SET measurements will enable clinicians to detect potentially life-threatening conditions earlier in all patients and clinical settings, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Jamie Arnold, APR
Public Relations Manager
Welch Allyn
315-685-4599
Dana Banks
Manager, Public Relations
Masimo Corporation
949-297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5, Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


New Published Study Finds Noninvasive Masimo PVI Accurately Predicts Fluid Responsiveness in Intensive Care Patients
PVI Predictive Ability Similar to Invasive Pulse Pressure Variation and Superior to Cardiac Output
Irvine, California – February 1, 2011 – Masimo (NASDAQ: MASI) announced today that a new study published in the peer-reviewed journal Critical Care Medicine demonstrates that noninvasive, continuous monitoring of Masimo Pleth Variability Index (PVI®) predicts fluid responsiveness with high sensitivity (95%) and specificity (91%) in mechanically-ventilated patients in the Intensive Care Unit (ICU).1 Multiple previous studies have shown that PVI predicts fluid responsiveness in surgical patients—helping clinicians improve fluid management to reduce patient risk,2,3,4 but this is the first published study demonstrating its effectiveness in predicting fluid responsiveness in critically-ill ICU patients.
Hemodynamic instabilityis a common problem for critically-ill patients, but the decision to administer fluid in an attempt to improve cardiac output is challenging. When necessary, fluid administration is critical to optimizing patient status and enabling end organ preservation, but unnecessary fluid administration is associated with increased morbidity and mortality. However, other dynamic indicators for assessing intravascular fluid volume are either invasive, operator dependent, costly, or unreliable predictors of whether a patient will benefit from fluid administration.
Citing numerous prospective studies showing that "only half of critically-ill patients respond to fluid boluses deemed necessary by attending physicians," researchers from CHU de Poitiers (France) acknowledged the "need for simple, noninvasive, and continuous bedside monitoring capable of detecting fluid responsiveness early in virtually all ICU patients, including those without an arterial catheter" in undertaking the current study. PVI provides clinicians with a noninvasive, continuous, and cost-effective measure for assessing whether patients will benefit from fluid administration—enabling clinicians to provide personalized and goal-directed fluid therapy1,2,3,4.
To evaluate the predictive ability of fluid responsiveness indicators, study researchers compared three indices in 40 mechanically-ventilated patients with circulatory insufficiency in the ICU. The indices ranged in level of difficulty to perform—from automatic (PVI, noninvasively obtained via the Masimo Radical-7 Pulse CO-Oximeter) to invasive (∆PP, respiratory variations in arterial pulse pressure obtained via arterial catheter) to operator-dependent (CO, cardiac output obtained via echocardiography). Each of the indices was recorded before and after fluid challenge, which consisted of either 500mL of 130/0.4 hydroxyethyl-starch (fluid) infused over 10 minutes with the patient in a semi-recumbent position (head at 45 degrees) or passive leg raising (PLR) if ∆PP <13%—indicating that the patient would be unlikely to respond to fluid administration.
Results showed 21 patients (19 fluid, 2 PLR) were responders—defined as an increase in cardiac output of >15% after fluid or PLR—and 19 patients (2 fluid, 17 PLR) were non-responders. A threshold value of 17% for PVI allowed discrimination between responders and non-responders at 95% sensitivity and 91% specificity and a ∆PP threshold value of 10% allowed discrimination between responders and non-responders at 100% sensitivity and 95% specificity. However, cardiac output (CO) was unable to reliably predict responders vs. non-responders. Additionally, responders had significantly higher average values vs. non-responders for PVI (28% vs. 11%) and ∆PP (22% vs. 5%) and both PVI (r=0.72, p<.0001) and ∆PP (r=0.77, p<.0001) correlated with the percentage change in CO induced by the fluid challenge—suggesting that "the higher PVI or ∆PP is at baseline, the higher the beneficial effect of volume expansion."
Finding that the "performance of PVI to predict fluid responsiveness was similar to that of ∆PP," researchers concluded that Masimo PVI "can predict fluid responsiveness in intensive care unit patients under mechanical ventilations." Researchers also noted the implications of using such dynamic fluid responsiveness predictors, commenting that "optimizing the patient's hemodynamic state with ∆PP or PVI monitoring has potential to decrease duration of hospital stay and mechanical ventilation, postoperative morbidity, and costs in patients undergoing high-risk surgery."
PVI is available as part of Masimo rainbow®SET platform—the first-and-only technology to noninvasively and continuously measure total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), perfusion index (PI), and acoustic respiration rate (RRa™), in addition to the 'gold standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), and pulse rate (PR).
1 Loupec T., Nandoumgar H., Frasca D., Petitpas F., Laksiri L., Baudouin D., Debaene B., Dahyot-Fizelier C., Mimoz O. "Pleth Variability Index Predicts Fluid Responsiveness in Critically-Ill Patients." Crit Care Med February 2011 Vol. 39, Issue 2, pp 294-299. For further reading, visit: http://journals.lww.com/ccmjournal/Abstract/2011/02000/Pleth_variability_index_predicts_fluid.8.aspx.
2 Cannesson M., Desebbe O., Rosamel P., Delannoy B., Robin J., Bastien O., Lehot JJ. "Pleth variability Index to Monitor the Respiratory Variations in the Pulse Oximeter Plethysmographic Waveform Amplitude and Predict Fluid Responsiveness in the Operating Theatre." British Journal of Anaesthesia August 2008; 101(2):200-6. For further reading, visit: http://www.ncbi.nlm.nih.gov/pubmed/18522935.
3 Zimmerman M., Feibicke T., Keyl C., Prasser C., Moritz S., Graf B., and Wiesenack C. "Accuracy of Stroke Volume Variation Compared with Pleth Variability Index to Predict Fluid Responsiveness in Mechanically-ventilated Patients Undergoing Major Surgery." European Journal of Anaesthesiology June 2010; 27(6):555-61. For further reading, visit: http://www.ncbi.nlm.nih.gov/pubmed/20035228.
4 Feissel M., Kalakhy R., Badie J., Robles G., Faller J., Teboul JL. "Plethysmography Variability Index: A New Fluid Responsiveness Parameter." Presented at the 29th International Symposium on Intensive Care and Emergency Medicine (ISICEM) Annual Meeting, March 25, 2009, Brussels, Belgium. For further reading, visit: http://ccforum.com/content/13/S1/P205.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo PVI is capable of detecting fluid responsiveness in virtually all ICU patients, and risks related to our assumptions that Masimo PVI monitoring has the potential to decrease hospital stay, mechanical ventilation, postoperative morbidity, and costs in patients undergoing high-risk surgery, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Phone: (949) 297-7348
Email: dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Provides Additional Comments Regarding Amendment to Settlement Agreement with Covidien
IRVINE, Calif., January 31, 2011 – Following today's announcement by Masimo (NASDAQ: MASI) that the company has amended its January 2006 settlement agreement with Covidien, Masimo provides the following additional information.
In conjunction with finalizing its 2010 financial results, Masimo is currently assessing the full financial impact of this amendment on its 2011 financial outlook. As part of this assessment, Masimo is considering the possibility of re-investing up to 50% of this incremental royalty revenue into its business in 2011. Masimo intends to provide 2011 guidance and discuss the impact of the amendment when the company announces its 2010 financial results.
Masimo announced last Friday that it will discuss its fourth quarter and full year 2010 financial results in a conference call and webcast on Tuesday, February 15, 2011, at 1:30 p.m. PT (4:30 p.m. ET).
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic MonitoringTM, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our amended royalty agreement. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce the anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations,
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.

Masimo and Covidien Announce Extension of Royalty Agreement
IRVINE, Calif., January 31, 2011 – Masimo (NASDAQ: MASI) and Covidien today announced that they have amended the existing settlement agreement initially reached in January 2006. Under the terms of the amendment, Covidien will pay Masimo a royalty of 7.75% for its current pulse oximetry products sold in the United States for three years, beginning March 15, 2011.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our amended royalty agreement. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce the anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations,
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Report Fourth Quarter and Full Year 2010 Financial Results after Market Close on February 15, 2011
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., January 28, 2011 -- Masimo (NASDAQ: MASI) announced today that it will release fourth quarter and full year 2010 financial results for the period ended January 1, 2011, after the market closes on February 15, 2011.
A conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 679-9937 for international callers. The reservation code for both dial-in numbers is 38781834. After the live webcast, the call will be available on Masimo's website through March 15, 2011. In addition, a telephonic replay of the call will be available through March 1, 2011. The replay dial-in numbers are (800) 642-1687 for domestic callers and +1 (706) 645-9291 for international callers. Please use reservation code 38781834.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Sheree Aronson
Vice President, Investor Relations,
Masimo Corporation
(949) 297-7043
saronson@masimo.com
Media Contact:
Dana Banks
Manager, Public Relations,
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


New Clinical Study Finds Masimo Noninvasive Hemoglobin Accurate in Patients with Critically Low Hemoglobin Levels
Study Presented at Society of Critical Care Medicine Demonstrates Masimo SpHb's Potential to Improve Blood Management Decisions in Severely Anemic Intensive Care Patients
Irvine, California – January 20, 2011 – Masimo (NASDAQ: MASI) announced today that a new study demonstrating the clinical accuracy and value of its noninvasive and continuous hemoglobin (SpHb®) monitoring technology breakthrough was presented this week at the Society of Critical Care Medicine (SCCM) Annual Critical Care Congress in San Diego. The largest multi-professional critical care event of the year, the SCCM Annual Congress reflects the latest in evidence-based research, clinical best practices, and medical developments that are shaping the future of critical care medicine.
The study, presented by researchers at Englewood Hospital and Medical Center (EHMC) in New Jersey, evaluated the accuracy of Masimo SpHb — a noninvasive measurement of hemoglobin blood levels obtained using a Masimo rainbow®ReSposable Sensor placed on the finger and continuously displayed on a Masimo Radical-7®Pulse CO-Oximeter™.SpHb measurements obtained from nine intensive care unit (ICU) patients with critically low hemoglobin levels (ranging from 4.3-8.6g/dL for Hb lab values) were compared with invasive blood samples drawn simultaneously and analyzed by a central laboratory. Results showed a mean bias and precision of 0.70 g/dL and 1.05 g/dL for SpHb, respectively, when compared to reference laboratory hemoglobin values—demonstrating "clinically acceptable agreement." Researchers concluded that "the ability to measure hemoglobin noninvasively and continuously has the potential to facilitate the timely detection of changes in hemoglobin and thus improve patient blood management decisions in patients with critically low hemoglobin."1
According to lead researcher, Anna Juhl, RN, Clinical Research Director of Anesthesiology and Critical Care at EHMC, "Two important elements of Patient Blood Management (PBM) are: 1) managing post-op blood loss with any resulting anemia, and 2) only transfusing based on clinical and lab guided evidence. The results of this study show that Masimo SpHb may help aid clinicians in achieving the goals of PBM in a timely fashion with less blood loss to the patient."
According to Dr. Michael O'Reilly, Chief Medical Officer at Masimo, "Successfully managing patients with very low hemoglobin levels is often a complex and risky undertaking that most physicians avoid by giving blood transfusions. However, the growing significance of deleterious transfusion effects and complications are now forcing physicians to re-evaluate transfusion decisions in critically ill patients. This study showcases Masimo SpHb's importance and unique role in aiding the clinical management of patients with very low hemoglobin levels—providing clinicians with a new tool to monitor and manage very low hemoglobin levels more effectively."
SpHb is available as part of the Masimo rainbow®SET platform—the first-and-only technology to noninvasively and continuously measure total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the 'gold-standard' Measure-Through Motion and Low Perfusion performance of Masimo SET®oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR).
1 Juhl A., Naqvi S., Aregbeyen O., Demir S., Shander A. "Accuracy of Noninvasive Hemoglobin Monitoring in Intensive Care Unit Patients with Very Low Hemoglobin." Englewood Hospital and Medical Center, Englewood, New Jersey. SCCM abstract #215 presented January 19, 2011.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, Pulse CO-Oximetry, Pulse CO-Oximeter, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57, Rad-8, Rad-5, Pronto-7, Pronto, Patient SafetyNet, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


New Johns Hopkins School of Medicine Study Shows Masimo Noninvasive Hemoglobin Monitoring is Accurate During Complex Spine Surgeries
Study Presented at Society for Technology in Anesthesia Demonstrates Masimo SpHb May Enable More Rapid Assessments of Patient Condition with Potentially Improved Blood Management Decisions
Irvine, California – January 18, 2011 – Masimo (NASDAQ: MASI) announced today that a new clinical study demonstrating the clinical accuracy of its noninvasive and continuous hemoglobin (SpHb®) monitoring technology breakthrough was presented last week at the Society for Technology in Anesthesia (STA) Annual Meeting.
The study, presented by the Johns Hopkins School of Medicine, evaluated the accuracy of Masimo SpHb—a noninvasive measurement of hemoglobin blood levels obtained using a Masimo Radical-7®Pulse CO-Oximeter™ with a Masimo rainbow®ReSposable Sensor. SpHb measurements obtained from 28 patients undergoing complex spine surgeries were compared with invasive blood samples drawn simultaneously and analyzed by a central laboratory. Results showed a mean bias of -0.3 for SpHb, a standard deviation of 1.0 g/dL and limits of agreement from -2.3 to 1.8 g/dL—demonstrating the "clinically acceptable accuracy" of SpHb when compared to a standard laboratory reference device. Researchers concluded that "continuous noninvasive SpHb monitoring may lead to earlier intervention and improved patient safety and care in the OR setting."1
According to lead researcher, Lauren Berkow, MD, Associate Professor of Anesthesiology and Critical Care Medicine at the Johns Hopkins School of Medicine, "The Masimo noninvasive hemoglobin monitor correlated strongly with gold-standard laboratory hemoglobin even during significant blood loss. The ability to continuously monitor hemoglobin during complex spine surgery may allow for earlier diagnosis and treatment of anemia related to surgical bleeding as well as prevent over-transfusion."
Traditional blood analysis has many drawbacks, including complexity, time-consuming turnaround times that can impact clinical decisions, and blood loss due to invasive blood draws that have been found to contribute substantially to the anemic conditions that commonly occur in critically ill patients—thereby increasing transfusion rates. Published studies have shown that transfusion of just one or two units of blood significantly increases infection, pneumonia, sepsis, and mortality after surgery.2-3 These studies also suggest that transfusions and their associated risks could be "largely avoided" through implementation of better blood management techniques and "more appropriate indicators" for transfusions.
The ability to noninvasively and continuously trend a patient's hemoglobin blood level during surgery allows for constant assessment of a patient's hemoglobin status, which may enable more appropriate clinical decisions—offering a breakthrough in blood management and patient safety that has the potential to improve patient outcomes, reduce patient exposure to unnecessary blood transfusions, and preserve precious blood resources.
According to Dr. Michael O'Reilly, Chief Medical Officer at Masimo, "Every surgical procedure bears certain risks—some may be preventable while others are unavoidable. Masimo SpHb fundamentally changes the way clinicians respond to bleeding and a patient's need for a blood transfusion by reducing measurement delays through real-time tracking and trending of hemoglobin blood levels. This study further underscores the value and impact of SpHb as a cost-effective solution to more accurately, easily, and rapidly monitor hemoglobin."
SpHb is available as part of the Masimo rainbow®SET platform—the first-and-only technology to noninvasively and continuously measure total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVI®), and acoustic respiration rate (RRa™), in addition to the 'gold-standard' Measure-Through Motion and Low Perfusion performance of Masimo SET® oxyhemoglobin (SpO2), perfusion index (PI), and pulse rate (PR).
1 Berkow L., Rotolo S., Mirski E. "Continuous and Noninvasive Hemoglobin Monitoring During Complex Spine Surgery." Johns Hopkins School of Medicine, Baltimore, Maryland. Presented at the STA Annual Meeting January 12-15, 2011.
2 Bernard AC et al. Intraoperative transfusion of 1U to 2U of packed red blood cells is associated with increased 30-day mortality, surgical site infection, pneumonia, and sepsis in general surgery patients. Journal of the American College of Surgeons. 2009;208:931-937.
3 Surgenor SD et al, for the Northern New England Cardiovascular Disease Study Group. The Association of Perioperative Red Blood Cell Transfusions and Decreased Long-Term Survival After Cardiac Surgery. Anesthesia & Analgesia 2009; 108:1741-1746.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies demonstrate Masimo SET provides the most reliable SpO2 and pulse rate measurements even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®SET Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow SET technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. Masimo SET and Masimo rainbow SET technologies can be also found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcomes and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Dana Banks
Masimo Corporation
(949) 297-7348
dbanks@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, Pulse CO-Oximetry, Pulse CO-Oximeter, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57, Rad-8, Rad-5, Pronto-7, Pronto, Patient SafetyNet, and SEDLine are trademarks or registered trademarks of Masimo Corporation.

