News & Media-2015
Home / News & Media / 2015
2015
NEWS & MEDIA:

Masimo Pledges to Increase Investment in Research & Development in Response to Today's Moratorium on the Medical Device Tax
Irvine, California – December 18, 2015 – Masimo (NASDAQ: MASI), the global innovator of noninvasive patient monitoring technologies, announced today that it will increase investment in research and development and other areas in response to the moratorium on the Medical Device Tax.
For years, the United States has led the world in life-saving medical innovations. Unfortunately, the Medical Device Tax of 2013 became an impediment to innovation that springs from start-ups. In addition, many established companies curtailed their investments in research and development and reduced their workforces to pay the new tax.
"We applaud the bipartisan efforts of Congress and the administration to suspend the medical device tax for two years," said Joe Kiani, Chairman and CEO of Masimo. "This two-year moratorium will allow us to begin to increase our investment in research and development and other areas."
@MasimoInnovates | #Masimo
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through-Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Media Contact:
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Retired U.S. Senator Tom Harkin Joins Masimo's Board of Directors
Irvine, California – December 17, 2015 – Masimo (NASDAQ: MASI), a global innovator of noninvasive patient monitoring technologies, announced today that retired Senator Tom Harkin, former five-term U.S. Senator and former Chairman of the Senate Health, Education, Labor and Pension (HELP) Committee, has been elected to Masimo's Board of Directors.
Senator Harkin retired from the U.S. Senate in January 2015. Senator Harkin was chairman of the Senate HELP Committee for eight years. In that role, he worked to ensure the health of our economy, guarantee access to quality education, and provide affordable, quality health care for all Americans. Senator Harkin crafted the prevention and wellness title of the country's new health reform bill, the Patient Protection and Affordable Care Act. Senator Harkin's signature legislation achievement was leading the effort and sponsorship of the Americans with Disabilities Act (ADA) signed into law in 1990 by President George H.W. Bush. He later created the Clinical Trials Network to measure the effectiveness of rehabilitation therapies for those living with paralysis, and drafted the Christopher and Dana Reeve Act, passed in 2009. Prior to his retirement, Senator Harkin worked closely with President Obama to lift restrictions on embryonic stem cell research.
"For years, Tom has worked tirelessly to ensure that everyone has access to affordable, quality health care and those who are in need are not forgotten. We are honored to welcome Tom to the Board," said Joe Kiani, Chairman and CEO of Masimo. "We believe his integrity, extensive experience and insight into our complex health system, along with his dedication and passion to help those in need will be extremely valuable to Masimo."
"I've been very impressed with Masimo's innovations and contributions to healthcare," said Harkin. "I got to know Joe through his advocacy work to improve competition in healthcare, align misaligned incentives that impede patient care and the Patient Safety Movement. He has been steadfast in his commitment to improve our healthcare system through innovation and advocacy. I look forward to working with him and the rest of the board to help Masimo with its mission to improve patient care and reduce cost of care."
Senator Harkin was first elected to the U.S. House of Representatives in 1974, and 10 years later, he was elected to US Senate. Senator Harkin was born in Cumming, Iowa, where he still resides with his wife Ruth. They have two daughters and three grandchildren.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through-Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Media Contact:
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


T.J. Samson is the First Hospital in Kentucky to Adopt Patient SafetyNet™
Irvine, California and Glasgow, Kentucky – December 1, 2015 – Masimo and T.J. Samson Community Hospital announced that the hospital is the first in Kentucky to install Masimo Patient SafetyNet, a remote monitoring and clinician notification system.
"Patient safety is my top priority," said Bud Wethington, Chief Executive Officer of T.J. Samson Community Hospital. "We did a lot of research in finding the system that was right for us. After trying several other systems and visiting another hospital that was utilizing Patient SafetyNet, we found it to be a perfect fit for our hospital. The installation was easier than anticipated and we were up and running in a very short amount of time."
Masimo Patient SafetyNet works in conjunction with Masimo beside monitors which provide continuous and non-invasive monitoring of oxygenation, pulse rate and respiration, and other parameters. When changes occur in the measured values, which may indicate deterioration in the patient's condition, in addition to bedside alerts, Patient SafetyNet automatically sends wireless alerts directly to clinicians. Continuous patient surveillance with Patient SafetyNet has been shown to reduce the need for rescue events and intensive care unit transfers in hospitals and, as a result, can reduce costs related to these events.1,2
"We are so impressed when we see a community hospital equip their team with the latest in technology to better monitor their patients and improve patient safety practices," said Joe Kiani, Founder and CEO of Masimo. "There are much larger hospitals throughout the country that have yet to install wireless notification systems for supplemental monitoring."
1 Taenzer A.H., Pyke J.B., McGrath S.P., Blike G.T. Anesthesiology. 2010 Feb;112(2):282-7.
2 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring - The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. Available online.
About T.J. Samson Community Hospital
Since 1929, T.J. Samson Community hospital has offered a variety of health services to the region, including acute care; preventive testing and treatment; community outreach activities; and partnerships with local heath care providers, civic organizations, and public schools. The hospital has undergone several expansions, including its most recent addition—a new wing to house an ultramodern labor and delivery floor, emergency department, cardiology laboratory, and intensive care unit. In May 1997, the hospital was designated as the site for one of Kentucky's congressionally mandated Family Practice Residency Programs and is now home to the University of Louisville Glasgow/Barren County Family Medicine Residency. Today, T.J. Samson Community Hospital is a 196-bed, acute-care facility, including sixteen skilled-care beds. And the T.J. Health Pavilion, providing outpatient services and physicians' offices. For more information, please visit www.tjsamson.org.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through-Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). The indications for EMMA include the following: 1) The EMMA Emergency Capnometer Monitor measures, displays and monitors carbon dioxide concentration and respiratory rate during anesthesia, recovery and respiratory care. It may be used in the operating suite, intensive care unit, patient room, clinic, emergency medicine and emergency transport settings for adult, pediatric and infant patients. 2) The EMMA Emergency Capnometer Analyzer measures and displays carbon dioxide concentration and respiratory rate during anesthesia, recovery and respiratory care. It may be used in the operating suite, intensive care unit, patient room, clinic, emergency medicine and emergency transport settings for adult, pediatric and infant patients. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®) contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Bart Logsdon
T.J.Samson
Phone: (270) 651-4618
Email: blogsdon@tjsamson.org
Irene Paigah
Masimo
Phone: (858) 616-8689
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Giving Back With Masimo
Irvine, California - November 25, 2015 - Masimo (NASDAQ: MASI) is grateful, as always, to have had a wonderful year – but we nonetheless feel deep sorrow at all of the suffering around us, from unnecessary patient harm to the many refugees displaced from war-stricken homelands, who face so many difficulties as they seek even the most basic human needs.
It is in this spirit that this year, rather than wait for the December holidays, we have decided to bring forward our traditional request to you, our loyal customers and friends, to help us donate to some of the worthiest charities and organizations: the need simply feels greater and more urgent this year. We have purposefully chosen a new list of recipients, centered on refugee assistance and patient safety. We simply ask that you respond with an email to charity@masimo.com, specifying your choice from the list below, and we will donate $10 in your name. We also encourage you to donate to them directly.
AidRefugees - https://www.whitehouse.gov/campaign/aidrefugees
Medecins Sans Frontieres (MSF) International (Doctors without Borders) - https://www.doctorswithoutborders.org/refugees
Patient Safety Movement Foundation - https://psmf.org/
RED CROSS - https://www.icrc.org/en/where-we-work/middle-east/syria
SAMS - Syrian American Medical Society - https://www.sams-usa.net/foundation/
SOS Children's Villages- http://www.sos-childrensvillages.org/
UN Refugee Agency - http://www.unhcr.org/emergency/5051e8cd6-560953162.html
UNICEF - https://www.unicef.org/emergencies/syrian-crisis
As a part of our commitment to giving back and to aiding refugees and providing quality health care for all, earlier this year Masimo announced a $5 million medical equipment donation to assist Jordan in providing healthcare to over 1 million Syrian refugees. A progress report video highlighting the Masimo relief effort was featured at the Clinton Global Initiative Annual Meeting in September in New York City, and can be viewed at the following link: https://youtu.be/8oQJqHoWTTg.
Thank you for being a part of our Masimo family. We are grateful to you all and wish you all a beautiful holiday season. Here's to a happier and healthier New Year!
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through-Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
- Media Contact:
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo to Present at 27th Annual Piper Jaffray Healthcare Conference
Irvine, California, November 24, 2015 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the 27th Annual Piper Jaffray Healthcare Conference at the New York Palace on Wednesday, December 2, 2015 at 2:00 p.m. Eastern Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion™ pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®) in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
- Investor Contact:
Eli Kammerman
Masimo
Phone: (858) 859-7001
Email: ekammerman@masimo.com - Media Contact:
Irene Paigah
Masimo
Phone: (858) 858-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.



India's Leading Maternity and Birthing Care Hospital Group, Cloudnine, Adopts Masimo SET® Technology to Screen Infants for CCHD
Bangalore, India & Irvine, California – November 16, 2015 – Masimo (NASDAQ: MASI) announced today that Cloudnine Hospital Group – a leader in providing premier quality healthcare to women and children in India – has upgraded system-wide to Masimo SET® pulse oximetry.
Congenital heart disease (CHD) is the most common birth defect and it is estimated that nearly one out of every 100 babies is born with CHD. It has been reported that around 78,000 infants die because of congenital heart disease in India every year.1 Critical congenital heart disease (CCHD), defined as CHD causing death or requiring invasive intervention in the neonatal period, occurs in one to two newborns per 1,000 live births.2
"We evaluated several monitors before selecting Masimo's superior SET® technology for all 16 Cloudnine Hospitals," said Dr. Kishore Kumar, Chairman and Senior Neonatologist, Cloudnine Hospitals. "Masimo SET® will enhance our existing protocols to provide superior screening and ensure that every baby born here now has the opportunity to live a healthy life. Our hope is that universal CCHD screening become mandatory for every child born in India."
"Early detection is key in this fragile patient population," said Joe Kiani, Founder and CEO of Masimo. "It's exciting to see the adoption of CCHD screening in India. Cloudnine's hospitals have the best in class morbidity and mortality outcomes in the country – 0% maternal mortality and a 99.83% survival rate for babies. When we developed SET®, we didn't realize how many wonderful new applications our Measure-Through Motion and Low Perfusion™ technology would make possible – so it's especially gratifying that we can help clinicians around the world save the lives of so many babies."
In a study of newborns, researchers have found CCHD was detected 63% of the time by standard screening and 83% of the time when screening with Masimo SET® was added.3 Over 100 million patients are monitored with Masimo SET® pulse oximeters around the world and in more than 100 comparative studies Masimo SET® outperformed other technologies.4,5 Researchers have found that Masimo SET® in combination with changes in clinical practice led to an increase of 31% in the detection of newborns with CCHD when used as a part of the CCHD screening protocol.3
Cloudnine hospitals are also the first in the country to make a formal patient safety commitment to the World Patient Safety Movement Foundation. This non-profit organization is dedicated to eradicating preventable patient deaths in hospitals around the world. With this step, Cloudnine joins an elite group of over 1,200 hospitals worldwide that have made public commitments to implementing Actionable Patient Safety Solutions in their respective organizations.
For more information on Masimo products, go to www.masimo.com.
For more information on the World Patient Safety Movement Foundation, go to www.patientsafetymovement.org.
- Report available at http://indianexpress.com/article/lifestyle/health/78000-infants-die-of-congenital-heart-disease-in-india-every-year-say-doctors/.
- Zhao QM, Ma XJ, Ge XL, Liu F, Yan WL, Wu L, Ye M, Liang XC, Zhang J, Gao Y, Jia B, Huang GY; Neonatal Congenital Heart Disease screening group. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. Lancet. 2014 Aug 30;384(9945):747-54.
- de-Wahl Granelli A, Wennergren M, Sandberg K, Mellander M, Bejlum C, Inganäs L, Eriksson M, Segerdahl N, Agren A, Ekman-Joelsson BM, Sunnegardh J, Verdicchio M, Ostman-Smith I. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009 Jan 8;338:a3037.
- Data on file.
- All studies available at https://professional.masimo.com/evidence/featured-studies/feature/.
About Cloudnine
Set up in 2007 by the renowned neonatologist Dr. R. Kishore Kumar, in partnership with investing partners Matrix Partners and Sequoia Capital, Cloudnine is India's premier destination for comprehensive maternal, gynecological, neonatal, and pediatric care. The organization's vision is to effectively bridge the gap between Indian and international standards through a combination of clinical excellence, comprehensive care and an atmosphere of well-being, to ensure world-class standards in woman and child healthcare.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through-Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®) contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
- Media Contact:
Irene Paigah
Masimo
Phone: (858) 616-8689
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


In New Study EMMA™ Capnograph Has Comparable Accuracy to Sidestream Capnography Against Gold-Standard Blood Gas Analysis
IRVINE, California – November 12, 2015 – Masimo (NASDAQ: MASI) announced today that in a new study that compared the end tidal carbon dioxide values (EtCO2) from both the Masimo EMMA capnograph and a conventional sidestream capnography device to the gold-standard carbon dioxide measurement, arterial blood gas (PaCO2), EMMA produced "reliable" values and had similar accuracy as sidestream capnography.1

EMMA capnograph from Masimo.
Carbon dioxide levels in the blood reflect the degree of gas exchange occurring in the lungs and provide a critical indicator of cardio-respiratory function that aids in the assessment of the adequacy of ventilation. PaCO2 is considered the gold standard to measure carbon dioxide levels but requires invasive blood sampling and laboratory analysis that can provide intermittent and delayed measurements. EtCO2 monitoring with capnography provides a noninvasive and continuous estimate of PaCO2 and has become a standard of care in the operating room and other critical care areas.2 Since 2010, the American Heart Association (AHA) has recommended the use of EtCO2 during cardiopulmonary resuscitation (CPR) for "confirmation and monitoring of endotracheal tube placement."3 Conventional capnography monitors require warm-up time to calibrate and measure EtCO2 from a sampling line connected to the breathing circuit. In contrast to bulkier and slower-to-calibrate sidestream capnography monitors, the EMMA is a compact and lightweight mainstream capnograph that clips directly onto the breathing circuit with no sampling line and minimal warm-up time.
The study, published in the Journal of Clinical Monitoring and Computing by Dr. Kyung Woo Kim and colleagues from Seoul Park Hospital, College of Medicine, Seoul, Korea, compared 100 EtCO2 values from both the EMMA and sidestream capnography (Datex Ohmeda S5 Anesthesia Monitor) in 35 patients undergoing general anesthesia to PaCO2 values measured from blood gas analysis (GEM Premier 3000) of simultaneously collected arterial blood samples. Compared to PaCO2, EMMA had a bias of 6.0 mm Hg with a percent error of 18% and sidestream capnography had a bias of 3.8 mm Hg with a percent error of 20%.
The authors concluded "EMMA capnometers are viable and lighter weight alternatives to legacy capnometers, as they produce capnographs and reliable EtCO2 values rapidly despite their small size and light weight." The authors also noted that "the data used in this study were only collected in situations of stable and normal respiration under general anesthesia," and "that the limitations affecting the prediction of the PaCO2 values are in accordance with the condition of the patient."
For more information on the EMMA capnograph, go to www.masimo.com.
- Kim (et al.), Comparison of end-tidal CO2 measured by transportable capnometer (EMMA capnograph) and arterial pCO2 in general anesthesia. J Clin Monit Comput 2015; published online.
- ASA Standards for Basic Anesthetic Monitoring. 2011.
- Field (et al.), Part 1: executive summary: 2010 American Heart Associated ion guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010; 122: S640-56.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through-Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). The indications for EMMA include the following: 1) The EMMA Emergency Capnometer Monitor measures, displays and monitors carbon dioxide concentration and respiratory rate during anesthesia, recovery and respiratory care. It may be used in the operating suite, intensive care unit, patient room, clinic, emergency medicine and emergency transport settings for adult, pediatric and infant patients. 2) The EMMA Emergency Capnometer Analyzer measures and displays carbon dioxide concentration and respiratory rate during anesthesia, recovery and respiratory care. It may be used in the operating suite, intensive care unit, patient room, clinic, emergency medicine and emergency transport settings for adult, pediatric and infant patients. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®) contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
- Media Contact:
Irene Paigah
Masimo
Phone: (858) 616-8689
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Reports Third Quarter 2015 Financial Results and Announces New Five Million Share Repurchase Plan
Q3 2015 Highlights (compared to Q3 2014):
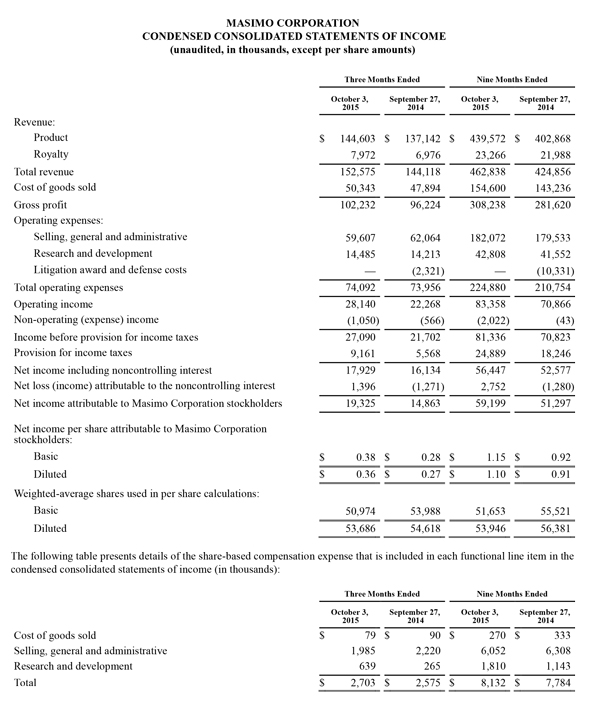
- Total revenue, including royalties, rose 5.9% to $152.6 million
- Product revenue rose 5.4% to $144.6 million
- Masimo rainbow® revenue rose 29.7% to $17.1 million
- SET® and rainbow® SET® units shipments were 45,800
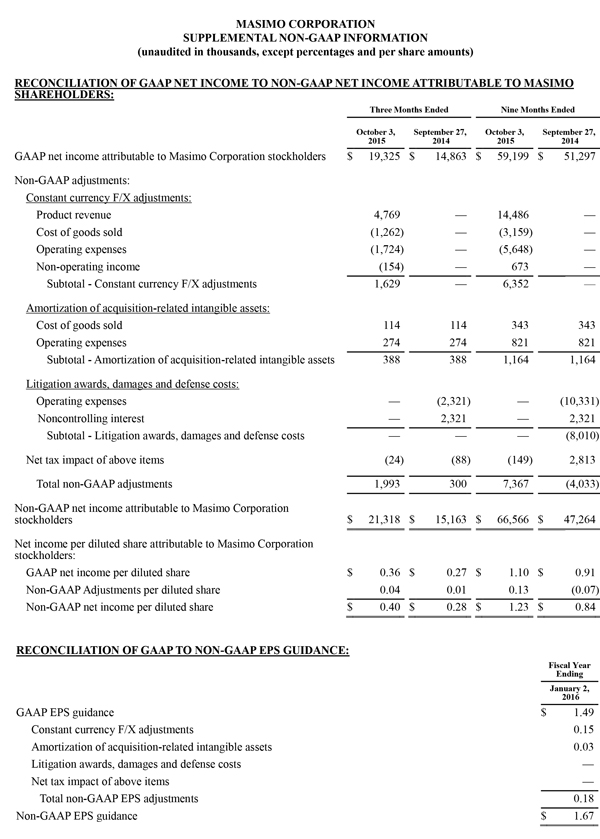
- GAAP net income of $19.3 million, or $0.36 per diluted share versus $14.9 million or $0.27 per diluted share in the year-ago period
- Non-GAAP net income of $21.3 million, or $0.40 per diluted share versus $15.2 million or $0.28 per diluted share in the year-ago period
Irvine, California, November 5, 2015 – Masimo (NASDAQ: MASI) today announced its financial results for the third quarter ended October 3, 2015.
Third quarter 2015 product revenues rose 5.4% to $144.6 million, compared to $137.1 million for the third quarter of fiscal year 2014, and total revenue, including royalties, rose 5.9% to $152.6 million, up from $144.1 million for the third quarter of fiscal year 2014. The unfavorable effect of foreign currency movements reduced third quarter product revenues by approximately $4.8 million.
The company's worldwide direct product revenue in the third quarter of 2015 rose by 3.5% compared to the same period in 2014 and represented 84.7% of product revenue. OEM sales, which accounted for 16.9% of product revenue, rose by 16.0% to $24.4 million in the third quarter of 2015 compared to the same period in 2014. Revenue from sales of Masimo rainbow® products rose by 29.7% to $17.1 million in the third quarter of 2015, compared to the same period in 2014.
GAAP net income for the third quarter of 2015 was $19.3 million, or $0.36 per diluted share, compared to GAAP net income of $14.9 million, or $0.27 per diluted share, in the third quarter of 2014. Non-GAAP net income for the third quarter was $21.3 million, or $0.40 per diluted share, versus non-GAAP net income of $15.2 million or $0.28 per diluted share in the year-ago period. During the third quarter of 2015, the company shipped 45,800 SET® pulse oximetry and rainbow® Pulse CO-Oximetry™ units, excluding handheld units. Masimo estimates its worldwide installed base as of October 3, 2015 to be 1,390,000 units, up 7.8% from 1,289,000 units as of September 27, 2014.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "Our third quarter results reflect the continued demand for our SET® Pulse Oximetry technology and our emerging rainbow® SET® Pulse CO-Oximetry technology. We are happy to be able to report record quarterly driver shipments and rainbow revenues, and another quarter of strong year-over-year earnings growth. Our underlying technology strength, along with our continued focus on value engineering and expense management, continue to make us confident about the financial leverage in our business model."
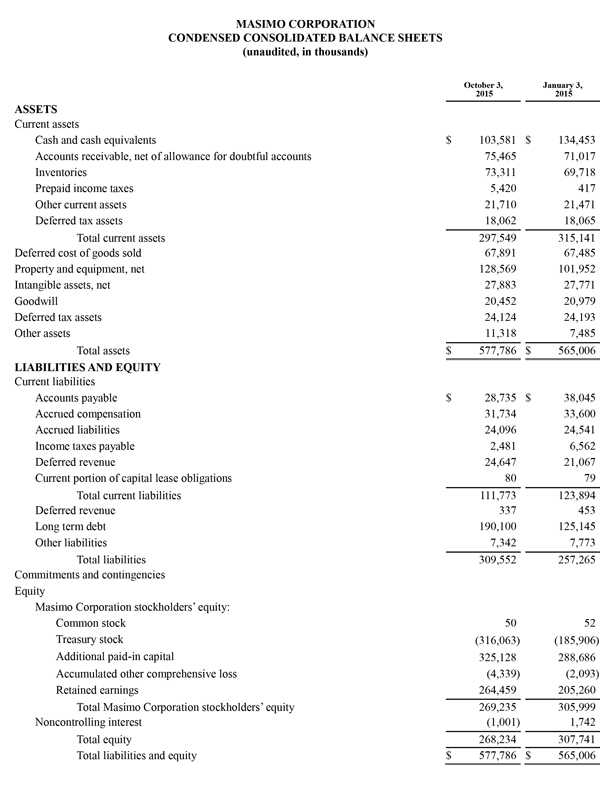
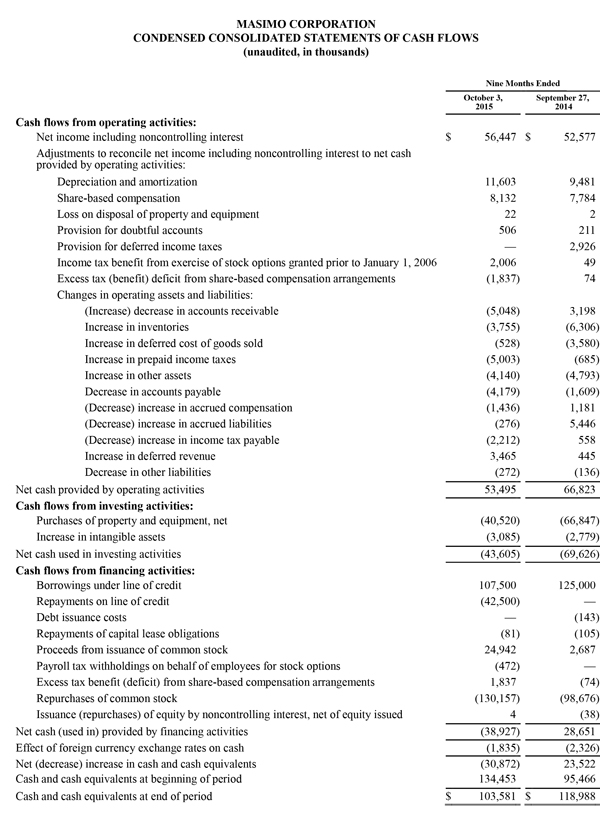
During the nine-month period ended October 3, 2015, the company generated $53.5 million in cash from operations and as of October 3, 2015, total cash and cash investments were $103.6 million compared to $134.5 million as of January 3, 2015. Also, during the nine-month period ended October 3, 2015, the company repurchased approximately 3.5 million shares of stock for $130.2 million, including approximately 1.2 million shares in the quarter ended October 3, 2015 for $48.5 million. These repurchases complete the original 9.0 million share stock repurchase plan authorized by the Board in 2013. Today, Masimo is also announcing the Board's authorization of a new 5.0 million share stock repurchase plan.
2015 Financial Guidance
Masimo today is updating its 2015 financial guidance. Masimo now expects fiscal 2015 total revenues to be approximately $627 million, up from $621 million. Total fiscal 2015 product revenues are now expected to be approximately $597 million, up from $592 million, while royalty revenues are now expected to be $30 million, up from $29 million. Masimo now also expects its fiscal 2015 GAAP earnings per diluted share to be $1.49, up from $1.43 and expects non-GAAP earnings per diluted share to be approximately $1.67, up from $1.61. Masimo will provide additional financial information during the conference call today. Each of the components of Masimo's guidance set forth above is an estimate only and actual performance could differ.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the call will be available online from the investor relations page of the company's website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 64862474. After the live webcast, the call will be available on Masimo's website through December 4, 2015. In addition, a telephonic replay of the call will be available through November 19, 2015. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 64862474.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our expectations for full fiscal year 2015 total and product revenues and GAAP and non-GAAP earnings per diluted share; demand for our products; anticipated revenue and earnings growth; our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies and reduce the cost of care; and demand for our technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET® and Masimo rainbow® SET® products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the amount and type of equity awards that we may grant to employees and service providers in the future; our ongoing litigation and related matters; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
- Investor Contact:
Eli Kammerman
Phone: (949) 297-7077
Email: ekammerman@masimo.com - Media Contact:
Irene Paigah
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation
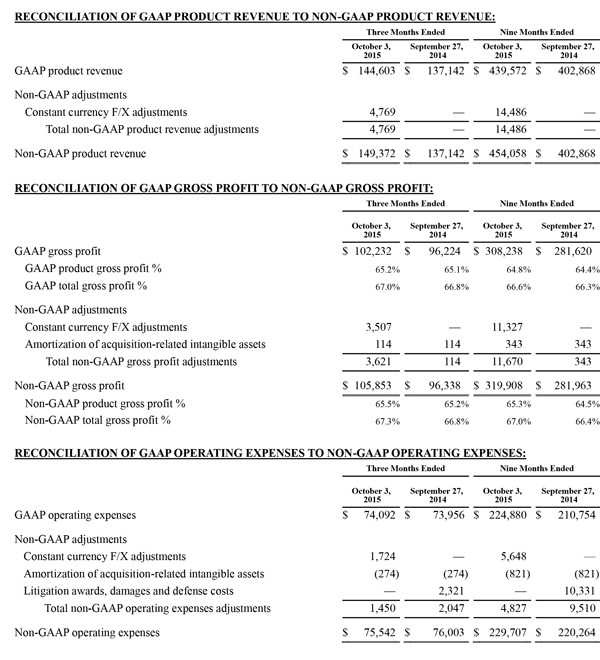
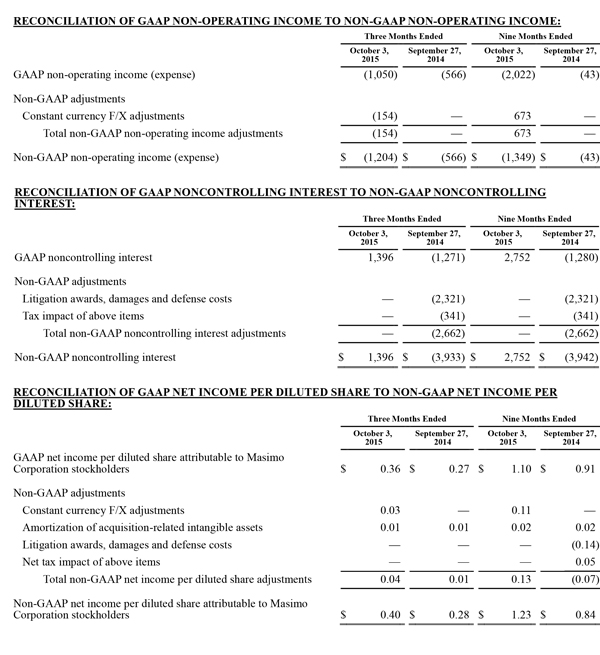
Non-GAAP Financial Measures
The non-GAAP financial measures contained herein are a supplement to the corresponding financial measures prepared in accordance with U.S. generally accepted accounting principles (GAAP). The non-GAAP financial measures presented exclude the items summarized in the above table that are more fully described below. Management believes that adjustments for these items assist investors in making comparisons of period-to-period operating results and that these items are not indicative of the company's ongoing core operating performance. These non-GAAP financial measures have certain limitations in that they do not reflect all of the costs associated with the operations of the company's business as determined in accordance with GAAP. Therefore, investors should consider non-GAAP financial measures in addition to, and not as a substitute for, or as superior to, measures of financial performance prepared in accordance with GAAP. The non-GAAP financial measures presented by the company may be different from the non-GAAP financial measures used by other companies.
The company has presented the following non-GAAP measures on a basis consistent with its historical presentation, to assist investors in understanding the company's core net operating results on an ongoing basis: (i) non-GAAP net income attributable to Masimo Corporation stockholders, (ii) non-GAAP product revenue, (iii) non-GAAP gross profit, (iv) non-GAAP operating expenses, (v) non-GAAP non-operating income (expense), (vi) non-GAAP noncontrolling interest, and (vii) non-GAAP net income per diluted share attributable to Masimo Corporation stockholders. These non-GAAP financial measures may also assist investors in making comparisons of the company's core operating results with those of other companies. Management believes non-GAAP product revenue, non-GAAP gross profit, non-GAAP net income attributable to Masimo Corporation stockholders and non-GAAP net income per diluted share attributable to Masimo Corporation stockholders are important measures in the evaluation of the Company's performance and uses these measures to better understand and evaluate our business.
The non-GAAP financial measures reflect adjustments for the following items, as well as the related income tax effects thereof:
Constant currency F/X adjustments. Some of our sales agreements with foreign customers provide for payment in currencies other than the U.S. Dollar. Similarly, certain of our product costs and operating expenses, and the related balance sheet payables and accruals, are denominated in foreign currencies other than the U.S. Dollar. These foreign currency revenues, costs and expenses, receivables, payables and accruals, when converted into U.S. Dollars, can vary significantly from period to period depending on the average and quarter-end exchange rates during a respective period. We believe that comparing these foreign currency denominated revenues, costs and expenses, receivables, payables and accruals by holding the exchange rates constant with the prior year period is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. We anticipate that fluctuations in foreign exchange rates and these related constant currency and other foreign exchange adjustments will continue to occur in future periods.
Amortization of acquisition-related intangibles. Amortization of intangibles generally represents costs incurred by an acquired company or other third party to build value prior to our acquisition of the intangible assets. As such, it is effectively part of the transaction costs of the acquisition rather than ongoing costs of operating our core business. As a result, we believe that exclusion of these costs in presenting non-GAAP financial measures provides management and investors a more effective means of evaluating its historical performance and projected costs and the potential for realizing cost efficiencies within our core business. Amortization of intangibles will recur in future periods.
Litigation awards, damages and defense costs. In connection with litigation proceedings arising in the course of our business, we have previously recorded expenses as a defendant in such proceedings in the form of damages and directly-related legal fees, as well as reversals of such damages and directly-related legal fee expenses upon a court vacating a prior award against us. In addition, we have also previously recorded recoveries of damages and directly-related legal fees as a plaintiff in litigation proceedings. We believe that exclusion of these expenses, expense reversals and recoveries is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. In this regard, we note that these expenses, expense reversals and recoveries are generally unrelated to our core business and/or infrequent in nature.
Lease termination exit costs. The effects of lease termination exits costs are excluded resulting from the purchase of the New Hampshire manufacturing facility that Masimo previous leased.
Reconciliations between the GAAP and non-GAAP amounts for each financial statement line item are as follows:


Two New Clinical Studies Using Masimo Technologies Presented at the American Society of Anesthesiologists Annual Meeting
Irvine, California – October 29, 2015 – Masimo (NASDAQ: MASI) announced today that two new studies using Masimo technologies were presented at the American Society of Anesthesiologists (ASA) annual meeting in San Diego, California, October 24-28. The conference is the largest gathering of anesthesiologists in the world.
Study Evaluating the EMMA Portable Capnometer in Children Under General Anesthesia
Carbon dioxide levels in the blood reflect the degree of gas exchange occurring in the lungs and provide a critical indictor of cardio-respiratory function that aids in the assessment of the adequacy of ventilation. In a study of 13 children (average age 18 months) undergoing surgery, Dr. Yuko Nawa and colleagues from the Hokkaido Medical Center for Child Health and Rehabilitation in Sapporo, Japan, compared the end tidal carbon dioxide values (EtCO2) from the Masimo EMMA portable capnometer and traditional sidestream capnography (GE Patient Monitor).1 Compared to sidestream capnography, the EMMA had 95% limits of agreement of -1.3 to 2.5 mm Hg, leading the investigators to conclude that the EMMA has "good correlation with sidestream type capnometer in children" and "may be useful for general anesthesia in out-of-operating room or in case of cardiopulmonary resuscitation, bedside respiratory care and patient transportation."
Study Evaluating Pleth Variability Index in Spontaneously Breathing Adults During Regional Anesthesia
Dexmedetomidine is an intravenous drug used to sedate patients during surgery which can also cause hypertension or hypotension that may increase patient risk. In a study of 42 spontaneously breathing patients under regional anesthesia,2 Dr. Makoto Sato and colleagues from Asahikawa Medical University in Hoikkado, Japan evaluated the association of baseline pleth variability index (PVI) from Masimo SET® pulse oximetry and dexmedetomidine-induced changes in blood pressure. A baseline PVI≤15 had a 94% sensitivity, 85% specificity, and area under the curve (AUC) of 0.93 (p=0.00002) for resulting hypertension. A baseline PVI≥16 had an 83% sensitivity, 64% specificity, and area under the curve (AUC) of 0.79 (p=0.0008) for resulting hypotension. The investigators concluded, "PVI can predict dexmedetomidine-induced changes in blood pressure in spontaneously breathing patients."
For more information on the Masimo technology, go to www.masimo.com.
- Nawa Y, Chaki T, Tamashiro K, Sato M, Mizuno E, Yamakage M. Accuracy of Portable Capnometer in Children. Proceedings of the American Society of Anesthesiologists, Oct. 27, 2015, San Diego, A4049, Room Upper 10.
- Sato M, Kunisawa T, Kurosawa A, Sasakawa T, Iwasaki H. Pulse oximeter-derived pleth variability index can predict dexmedetomidine-induced changes in blood pressure in spontaneously breathing patients. Proceedings of the American Society of Anesthesiologists, Oct. 26, 2015, San Diego, A3153, Hall B2, Area B.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). For the U.S. market, PVI is a measure of dynamic changes in the perfusion index (PI) that occur during the respiratory cycle. The PVI calculation is accomplished by measuring changes in PI over a time interval where one or more complete respiratory cycles have occurred. PVI is displayed as a percent (0-100%). PVI may show changes that reflect physiologic factors such as vascular tone, circulating blood volume and intrathoracic pressure excursions. The utility of PVI is unknown at this time and requires further clinical studies. Technical factors that may affect PVI include probe malposition and patient motion. The indications for EMMA include the following: 1) The EMMA Emergency Capnometer Monitor measures, displays and monitors carbon dioxide concentration and respiratory rate during anesthesia, recovery and respiratory care. It may be used in the operating suite, intensive care unit, patient room, clinic, emergency medicine and emergency transport settings for adult, pediatric and infant patients. 2) The EMMA Emergency Capnometer Analyzer measures and displays carbon dioxide concentration and respiratory rate during anesthesia, recovery and respiratory care. It may be used in the operating suite, intensive care unit, patient room, clinic, emergency medicine and emergency transport settings for adult, pediatric and infant patients. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®) contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
- Media Contact:
Irene Paigah
Masimo
Phone: (858) 616-8689
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces FDA 510(k) Clearance for MightySat™ Rx Fingertip Pulse Oximeter
Demonstrations held at the ASA Anesthesiology 2015 Conference in Masimo Booth 3437 - San Diego Convention Center on October 24-28, 2015
Irvine, California – October 27, 2015 – Masimo (NASDAQ: MASI) announced today FDA 510(k) clearance for MightySat Rx, a fingertip pulse oximeter that incorporates Masimo SET® Measure-through Motion and Low Perfusion™ technology. 
MightySat Rx features the same Masimo SET® technology that is used in leading hospitals worldwide and is present in all Masimo bedside devices and in many leading multi-parameter monitors. The MightySat noninvasively measures arterial oxygen saturation (SpO2) and pulse rate (PR), in addition to perfusion index (PI) and optional pleth variability index (PVI®). Masimo SET® technology enables accurate SpO2 and pulse rate measurements during motion and low perfusion.
"We use Masimo SET® throughout our hospital, and now MightySat Rx allows me to use Masimo SET® technology in a fingertip pulse oximeter - without compromising on performance," said Jon Carlson, RT, RRT-NPS, Director of Respiratory Care Services at Mercy Hospital of Buffalo, New York. "Its portability, durability, and convenient, small size are key. By pairing the device with my iPhone, I can easily view my patients' SpO2 and pulse rate trends."
MightySat Rx is designed to comfortably grip a patient's finger using a flexible, conforming silicon pad. An OLED display presents information clearly and a touchpad allows for customization. MightySat Rx is designed to be both rugged and lightweight.
"We are excited to provide clinicians the same accuracy provided by our other monitors in a fingertip pulse oximeter, said Joe Kiani, Founder and CEO of Masimo. "The MightySat Rx is an impressive device in a very compact form factor that provides clinicians easy access to patient data."
MightySat Rx is available in three versions: MightySat Rx, MightySat Rx with Bluetooth LE, and MightySat Rx with Bluetooth LE & PVI. The versions with Bluetooth allow iOS and Android™ mobile devices to display, trend, and communicate the measurements made on MightySat, using the free, downloadable Masimo Professional Health app.
For more information on MightySat Rx, go to www.masimo.com/pulseOximeter/mightysatRx.htm.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®) contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
- Media Contact:
Irene Paigah
Masimo
Phone: (858) 616-8689
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo & Atheer to Demonstrate Prototype of Interactive Root® Iris™ Display Powered by AiR™ Smart Glasses Platform
Demonstrations held at the ASA Anesthesiology 2015 Conference in Masimo Booth 3437 - San Diego Convention Center on October 24-28, 2015
IRVINE, California – October 22, 2015 – Masimo (NASDAQ: MASI) and Atheer announced today that they will unveil a prototype of the Root Iris display App using the Atheer AiR (Augmented interactive Reality) platform and AiR Glasses at the American Society of Anesthesiologists (ASA) annual conference. Demonstrations will be held at Masimo's Booth 3437 at the San Diego Convention Center on October 24-28, 2015. The demonstrations will allow conference attendees to experience a prototype display and touch-free interactions powered by the Atheer AiR Glasses that, if cleared by the FDA, would provide clinicians with a novel display and interaction experience. Attendees will be invited to provide feedback on features, usability and requirements.
"This is the future of information display and interaction," said Joe Kiani, Founder and CEO of Masimo. "What was once seen only in the movies is now on the way to becoming a reality. When combined with our Root Iris technology, AiR Glasses may in the future empower anesthesiologists to visualize data from multiple information sources and interact with the data using motion gestures while continuing to care for the patient."
"We are delighted to partner with Masimo – one of the most innovative medical technology companies in the world. Our AiR smart glasses platform is known for its rich display as well as unique touch-free interaction using gestures, voice and head motion. Now both our companies are leveraging our strengths to develop a novel platform for the medical community," said Alberto Torres, CEO of Atheer.
Special demonstrations and interviews will be scheduled by appointment for the media. To schedule a media demonstration and interview time, please call (858) 859-7001. To obtain a media pass for the Anesthesiology 2015 conference, please email pr@asahq.org or call (847) 268-9106.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through-Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
About Atheer
Atheer is the pioneer of AiR™ (Augmented interactive Reality) computing that is designed to enhance deskless professional productivity. The award-winning Atheer AiR platform combines the power of immersive augmented reality with intelligent gesture, voice and head-motion interaction, all on a mobile, smart glasses platform. Wearing our see-through AiR Glasses, you can view rich information critical to your workflow, interact with the information naturally and collaborate with your remote peers like never before – all while on the move, without the need to hold any device. Leveraging the Atheer AiR platform, developers and companies around the world are creating next generation enterprise productivity applications and workflows to empower the 21st century workforce. For more information, please visit www.atheerlabs.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®) contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
- Media Contact:
Irene Paigah
Masimo
Phone: (858) 858-7001
Email: irenep@masimo.com - Media Contact:
Kristen Maynard
Atheer
Phone: (415) 350-4147
Email: kristen.maynard@sparkpr.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo to Report Third Quarter 2015 Financial Results after Market Close on Thursday, November 5
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., October 21, 2015 -- Masimo (NASDAQ: MASI) announced today that it will release financial results for the third quarter ended October 3, 2015, after the market closes on Thursday, November 5, 2015. The conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be available online from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 64862474. After the live webcast, the call will be available on Masimo's website through December 4, 2015. In addition, a telephonic replay of the call will be available through November 19, 2015. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 64862474.
@MasimoInnovates || #Masimo
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion™ pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Eli Kammerman
Masimo
Phone: (949) 297-7077
Email: ekammerman@masimo.com
Media Contact:
Irene Paigah
Masimo
Phone: (858) 858-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Dr. Steven Barker Awarded the 2015 IAMPOV Lifetime Achievement Award
IRVINE, California – October 14, 2015 - Masimo (NASDAQ: MASI) announced today that a member of its Board of Directors, Chairman of its Scientific Advisory Board, and its Chief Science Officer, Dr. Steven Barker, Ph.D., M.D., was honored with the Harvey W. Weiley Lifetime Achievement Award at this month's Innovations and Applications of Monitoring Perfusion, Oxygenation, and Ventilation (IAMPOV) Symposium, in Tokyo, Japan.
"We are pleased to recognize Dr. Steven Barker as the winner of the 2015 IAMPOV Lifetime Achievement Award," said Dr. Katsuyuki Miyasaka, IAMPOV Symposium Chair. "Dr. Barker was selected based on his lifelong work in championing the development of vitally important monitoring technologies and associated testing."
The IAMPOV Symposium brings together clinicians, device developers, and researchers for an interchange of expertise and ideas to promote the development of better healthcare monitoring technologies and to improve their application in the areas of perfusion, oxygenation, and ventilation.
"We congratulate Dr. Steven Barker for this Lifetime Achievement award," said Joe Kiani, Founder and CEO of Masimo. "Steve has been on the cutting edge of research in the areas of oxygen transport, oxygen saturation monitoring, dyshemoglobins, brain function monitoring, and many other patient monitoring modalities. His steadfast commitment to science and advancements in the area of patient care is second to none. Steve's work has helped shed light on the problems and advancements in the area of oxygen transport."
Steven J. Barker, PhD, MD, received his PhD in Aeronautical Engineering from the California Institute of Technology in 1972, and his MD from the University of Miami in 1981. He has reached the rank of tenured professor in both engineering (University of California at Los Angeles) and anesthesiology (University of California at Irvine, University of Arizona). He chaired the Department of Anesthesiology at UC Irvine from 1990 to 1995, and then at the University of Arizona from 1995 to 2013. He has published over 150 scholarly works, including 15 textbook chapters. Dr. Barker has served as president of the national organization of anesthesiology department chairs (AAPD) and of the Society for Technology in Anesthesia (STA), senior oral examiner for the American Board of Anesthesiology, and Section Editor for Technology for the journal Anesthesia and Analgesia. During his 18-year tenure as anesthesiology chair at the University of Arizona, Dr. Barker was active in a number of governance roles outside his department. He also served seven years as the American Society of Anesthesiologists (ASA) Director for Academic Anesthesiology, representing all university anesthesiology departments for ASA. Since 2005, Dr. Barker has been a member of Masimo's Board of Directors and Chairman of Masimo's Scientific Advisory Board. In 2015, Dr. Barker also took on the role of Chief Science Officer of Masimo.
@MasimoInnovates || #Masimo
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through-Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Media Contact:
Irene Paigah
Masimo
Phone: (858) 858-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo PVI® Evaluated in 18-Study Meta-Analysis For Ability to Help Clinicians Assess Fluid Responsiveness
NEUCHATEL, Switzerland – October 8, 2015 – Masimo (NASDAQ: MASI) announced today that an 18-study meta-analysis of 665 subjects reported that Masimo's Pleth Variability Index (PVI®), available with Masimo's SET® and rainbow® SET® monitoring platforms, may help clinicians assess fluid responsiveness in mechanically ventilated patients in normal sinus rhythm in the operating room and intensive care unit.1
Clinicians commonly use intravenous fluid administration in the operating room and intensive care unit to attempt to improve blood flow, or cardiac output.2 Administering either too little fluid or too much fluid can increase patient risk.2 However, traditional "static" monitoring parameters such as central venous pressure (CVP) are considered unreliable to assess fluid responsiveness.2 Therefore, experts recommend the use of "dynamic" parameters that measure physiologic variation over the respiratory cycle.3 Multiple dynamic parameters have been shown to help clinicians assess fluid responsiveness, but most dynamic parameters require invasive, complex, and/or costly methods. PVI is a measure of the dynamic changes in the Perfusion Index (PI) that occur during one or more complete respiratory cycles, and may show changes that reflect physiologic factors such as vascular tone, circulating blood volume, and intrathoracic pressure excursions. In contrast to other dynamic parameters, PVI is noninvasive, easily obtained with any Masimo SET® or rainbow® sensor, and has no incremental procedural cost.
The meta-analysis, published in the Journal of Clinical Monitoring and Computing by Dr. Chu and colleagues from The First Hospital of China Medical University, Shenyang, China, combined the findings from 18 independent studies evaluating the ability of PVI to help clinicians assess fluid responsiveness. The authors reported that the pooled area under the curve (AUC) for PVI to correctly determine fluid responsiveness was 0.88 (95% confidence interval 0.84 to 0.91). The pooled sensitivity of PVI, or its ability to determine whether cardiac output would increase upon fluid administration, was 0.73 (95% confidence interval 0.68 to 0.78). The pooled specificity of PVI, or its ability to determine whether cardiac output would not increase upon fluid administration, was 0.82 (95% confidence interval 0.77 to 0.86). The authors noted that the sensitivity of PVI was greater in the operating room than in the intensive care unit (0.84 vs. 0.56, p=0.00004). The authors also noted that "the applicability of PVI may be limited by potential interference from several factors such as spontaneous breathing activity, arrhythmia, and low peripheral perfusion." Technical factors that may affect PVI include probe malposition and patient motion.
For more information on PVI, go to www.masimo.com.
@MasimoInnovates || #Masimo
- Chu (et al.). Accuracy of pleth variability index to predict fluid responsiveness in mechanically ventilated patients: a systematic review and meta-analysis: J Clin Monit Comput. 2015, 8:5.
- Navarro (et al.). Perioperative fluid therapy: a statement from the international Fluid Optimization Group. Perioperative Medicine. 2015, 4:3.
- Vallet B (et al.). Strategy for perioperative vascular filling - Guidelines for perioperative haemodynamic optimization. Experts' Formalized Recommendations, French Society of Anaesthesia and Intensive Care (SFAR), Validation by the administrative council of SFAR on 19 October 2012.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through-Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com. For the U.S. market, PVI is a measure of dynamic changes in the perfusion index (PI) that occur during the respiratory cycle. The PVI calculation is accomplished by measuring changes in PI over a time interval where one or more complete respiratory cycles have occurred. PVI is displayed as a percent (0-100%). PVI may show changes that reflect physiologic factors such as vascular tone, circulating blood volume and intrathoracic pressure excursions. The utility of PVI is unknown at this time and requires further clinical studies. Technical factors that may affect PVI include probe malposition and patient motion.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®) contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Irene Paigah
Masimo
Phone: (858) 858-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Donates $5 Million in Medical Equipment to Assist Jordan in Providing Healthcare to Over 1 Million Syrian Refugees
Relief Effort Progress Highlighted at the Clinton Global Initiative Annual Meeting in New York,
September 26-29, 2015
IRVINE, California – September 27, 2015 – Masimo (NASDAQ: MASI) announced progress today at the 2015 Clinton Global Initiative Annual Meeting towards its commitment to donate $5 million in Signal Extraction Technology® Pulse Oximeters and Pulse CO-Oximeters® and other medical equipment to Jordanian hospitals to help improve patient care for Syrian and Iraqi refugees, as well as Jordanian citizens. A progress report video highlighting the Masimo relief effort was featured at the Plenary Session called Investing in Prevention and Resilient Health Systems. The first shipment of equipment is already in use at Al-Bashir hospital in Amman. The rest of the equipment will arrive at the Ministry of Health Office in Jordan shortly. Masimo also committed to provide ongoing training and technical support to the clinicians in Jordan.
The wars in Syria and Iraq are one of the largest ongoing humanitarian crises in the world, with over an estimated million people seeking refuge now living within Jordan's borders, more than 600,000 of whom are registered with the United Nations High Commissioner for Refugees (UNHCR). Managing healthcare is a critical requirement of stability during crises and, while new hospitals are being built near refugee camps, the vast majority of refugees reside in existing communities, heightening the demand for health services throughout Jordan. The significant burden of providing healthcare services both for routine and emergency needs is worsening daily.
"The people of Jordan have opened their hearts and country to help their neighbors in need," said Joe Kiani, Founder and CEO of Masimo. "The situation in Syria keeps getting worse. The people of Jordan are our heroes – taking in the Palestinian refugees, the Iraqi refugees, then the Egyptian refugees, and now the large influx of Syrian refugees. We knew our plan for aid needed to be long-term. We not only donated the much-needed oximeters, other medical equipment, and supplies but we also committed to train and provide continued technical support for the Jordanian clinicians that assist in the front line care of the people of Jordan. The need is great and it is too overwhelming for just a few countries to shoulder. As global citizens, we all need to get involved and help the people of Syria and those trying to save them."
Masimo's commitment to Jordan is one of several ongoing CGI commitments, and was made at a roundtable meeting convened by CGI in 2014, at which President Clinton and his Majesty King Abdullah Il ibn Al Hussein of Jordan met with Masimo's Joe Kiani, among others, to discuss the humanitarian crisis.
The measurements provided by pulse oximeters are often referred to as the "fifth vital sign." In a report published by the Jordanian government, they are identified as one of the most needed pieces of hospital equipment. Pulse oximeters allow a health worker to monitor a person's oxygen levels. Confirming oxygen levels in the blood is critical as it helps identify those in need of care, those who are deteriorating, and those who are not. Pulse oximeters are widely used from health clinics to emergency departments, from surgery and the intensive care unit to the general floor. Over 100 million patients are monitored with Masimo SET® pulse oximeters around the world and in more than 100 comparative studies Masimo SET® outperformed other technologies. Researchers have found that Masimo SET® in combination with changes in clinical practice led to a reduction in retinopathy of prematurity (ROP) in preterm babies in the neonatal intensive care unit (NICU)1 and an increase of 31% in the detection of newborns with critical congenital heart disease (CCHD) when used as a part of the CCHD screening protocol.2 Researchers found improved outcomes3 following the installation of continuous patient monitoring with Masimo SET® on post-surgical patients, including that zero patients suffered brain damage or died over a five-year period.4 A recent study with a Masimo Pulse CO-Oximeter® (SpHb and PVI) also showed that 30-day mortality after surgeries dropped by 25%.5
- Castillo A, Deulofeut R, Critz A, Sola A. Prevention of retinopathy of prematurity in preterm infants through changes in clinical practice and SpO2 technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A, Wennergren M, Sandberg K, Mellander M, Bejlum C, Inganäs L, Eriksson M, Segerdahl N, Agren A, Ekman-Joelsson BM, Sunnegardh J, Verdicchio M, Ostman-Smith I. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009 Jan 8;338:a3037.
- Taenzer AH, Pyke JB, McGrath SP, Blike GT. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010 Feb;112(2):282-7.
- Taenzer et al. The Dartmouth Experience. APSF Newsletter. Spring-Summer 2012. Volume 27, No. 1, 1-28.
- Ponsonnard S, Yonnet S, Marin B, Cros J, Ben Miled S, Nathan N. "Continuous Hb and plethysmography variability index (PVI) monitoring is associated to a decreased mortality at the scale of a whole hospital." Proceedings of the European Society of Anaesthesiology's Euroanaesthesia 2015 Annual Congress, May 30-June 2, Berlin, Germany, 16AP3-2, Room A1 – Poster Abstract Presentation Session, e-Board 8.
@MasimoInnovates || #Masimo
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies. Our mission is to improve patient outcomes and reduce the cost of care by taking noninvasive monitoring to new sites and applications. In 1995, the company debuted Masimo SET® Measure-through-Motion and Low Perfusion™ pulse oximetry, which has been shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms. The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and it is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
About the Clinton Global Initiative
Established in 2005 by President Bill Clinton, the Clinton Global Initiative (CGI), an initiative of the Clinton Foundation, convenes global leaders to create and implement solutions to the world's most pressing challenges. CGI Annual Meetings have brought together 190 sitting and former heads of state, more than 20 Nobel Prize laureates, and hundreds of leading CEOs, heads of foundations and NGOs, major philanthropists, and members of the media. To date, members of the CGI community have made more than 3,200 Commitments to Action, which have improved the lives of over 430 million people in more than 180 countries. In addition to the Annual Meeting, CGI convenes CGI America, a meeting focused on collaborative solutions to economic recovery in the United States; and CGI University (CGI U), which brings together undergraduate and graduate students to address pressing challenges in their community or around the world. This year, CGI also convened CGI Middle East & Africa, which brought together leaders across sectors to take action on pressing social, economic, and environmental challenges.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®) contributes to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable
- Media Contact:
Irene Paigah
Masimo
Phone: (858) 616-8689
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.



Ranken Jordan Pediatric Bridge Hospital Upgrades their Monitors to the Radical-7® Featuring Masimo SET® Pulse Oximetry
MARYLAND HEIGHTS, Missouri, and IRVINE, California – September 14, 2015 - Masimo (NASDAQ: MASI) today announced that Ranken Jordan Pediatric Bridge Hospital has upgraded their monitors to the Radical-7 featuring Masimo SET® pulse oximetry.
"For children with complex medical conditions such as brain injuries, congenital defects or complications due to premature birth, our hospital specializes in bridging the gap between traditional hospital treatments and going home," said Laureen K. Tanner, RN, MSN, FACHE, President and Chief Executive Officer of Ranken Jordan Pediatric Bridge Hospital. "Breakthrough medical technology like this helps our team make the impossible possible on a daily basis."
The Radical-7 features Masimo SET® Measure-through-Motion and Low Perfusion™ pulse oximetry, shown in multiple studies to significantly reduce false alarms and accurately monitor for true alarms.1,2 The benefits of Masimo SET® have been proven in more than 100 independent and objective studies and used on more than 100 million patients in leading hospitals.3,4 Researchers have found that Masimo SET®, in combination with changes in clinical practice, led to a reduction in severe retinopathy of prematurity (ROP) in pre-term infants.5 In a study of newborns, researchers have found critical congenital heart disease (CCHD) was detected 63% of the time by standard screening and 83% of the time when screening with Masimo SET® was added.6 The Radical-7 allows hospitals the ability to upgrade their monitor with other breakthrough measurements that are only available with Masimo rainbow® technology.
"The amazing team at Ranken Jordan Pediatric Bridge Hospital has been taking care of children with complex needs for over 70 years. They are the only pediatric sub-acute facility in Missouri and one of the largest in the Midwest," stated Joe Kiani, Founder and CEO of Masimo. "Performance in challenging situations is key and we are happy that their clinical team now has access to our flagship monitor. It is our privilege to help facilities like Ranken Jordan help kids and families safely transition from the acute care hospital to home."
@MasimoInnovates || #Masimo
1 Barker SJ. Anesth Analg. 2002 Oct;95(4):967-72
2 Shah N et al. J. Clin Anesth. 2012 Aug; 24(5):385-91
3 All studies available at http://www.masimo.com/cpub/clinical-evidence.htm
4 Data on file
5 Castillo A et al. Acta Paediatr. 2011 Feb;100(2):188-92
6 de-Wahl Granelli AD et al. BMJ. 2009;338

About Ranken Jordan Pediatric Bridge Hospital
Ranken Jordan offers care through 62,000 square-foot, a 34-bed inpatient program and several outpatient programs, including outpatient therapy, intensive day treatment, a physiatry clinic and a comprehensive orthopedic rehabilitation clinic. The current hospital building houses ultramodern equipment and is specifically designed to entire patients to get out of their rooms and engage in daily life-centered activities. Ranken Jordan's Community Program provides free recreational and educational programs for all kids and their families. For more information, contact us or call (314) 872-6400. www.rankenjordan.org
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-through Motion and Low Perfusion™ pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In the U.S., Masimo rainbow® SET pulse CO-OximeterTM instruments and sensors are indicated to provide noninvasive monitoring of arterial oxygen saturation (SpO2) and pulse rate (PR), carboxyhemoglobin saturation (SpCO®), methemoglobin saturation (SpMet®), total hemoglobin concentration (g/dl SpHb), and/or respiration rate (RRa®). The devices are not intended to replace laboratory measurements of hemoglobin and have not been cleared by FDA to detect bleeding or reduce the amount or frequency of red blood cell (RBC) transfusions. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®) contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Irene Paigah
Masimo
Phone: (858) 858-7001
Email: irenep@masimo.com
Tyler Mathews
RankenJordan
Phone: (314) 872-6503
Email: tyler.mathews@rankenjordan.org
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo to Present at Morgan Stanley Global Healthcare Conference
Irvine, California, September 8, 2015 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the Morgan Stanley Global Healthcare Conference at the Grand Hyatt New York on Friday, September 18, 2015, at 11:05 a.m. Eastern Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion™ pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®) in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
- Investor Contact:
Eli Kammerman
Masimo
Phone: (949) 297-7077
Email: ekammerman@masimo.com - Media Contact:
Irene Paigah
Masimo
Phone: (858) 858-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


![]()
Masimo Partners with St. Joseph Hoag Health to Open a Wellness Center for Employees inside their Headquarters
New Wellness Center is the latest edition of St. Joseph Hoag Health's pioneering integrated medical care and lifestyle management for worksite wellness
(August 26, 2015, IRVINE, CALIF.) – Expanding on St. Joseph Hoag Health's successful efforts to bring health and wellness services directly into the workplaces, Masimo (NASDAQ: MASI) has teamed with the integrated health care system to bring a new wellness center to its worldwide headquarters at 52 Discovery in Irvine, California. The new Masimo Wellness Center will offer wellness services beginning August 25, 2015 and medical services will begin in September 2015. The center is staffed daily by a full-time wellness coach and registered nurse.
The center will serve 550 employees located in Irvine. The walk-in facility offers integrated wellness and medical services ranging from health coaching, education, and screenings to onsite lab draws and flu shots. The center uniquely features telehealth that allows for primary episodic care for common illnesses and conditions, all delivered virtually through St. Joseph Hoag Health's telemedicine network. St. Joseph Hoag Health manages the center and provides clinical and operations expertise.
"We are excited about this new addition to our wellness program," stated Joe Kiani, Founder and CEO of Masimo. "Our team consists of talented individuals who are passionate about saving lives. They go above and beyond each and every day. This new wellness center is designed to help our team stay healthy. When we looked at offering medical services onsite, we immediately thought of St. Joseph Hoag Health."
"Access and convenience are cited as top reasons why employees don't seek health care – it just doesn't fit readily into busy daily routines," said Richard Afable, MD, MPH, president & CEO of St Joseph Hoag Health. "St. Joseph Hoag Health is committed to partnering with companies to improve workforce health through the effective management of overall costs."
"Our collaboration with Masimo is another exciting step forward in our commitment to help people live healthier lives by being more available to them where they work and live," said Annette Walker, Executive Vice President of Strategic Services for St. Joseph Hoag Health. "The wellness center concept is about connecting with people within the ordinary patterns of their lives. Doing so provides powerful opportunities for us to make it easier for individuals – and businesses and our communities – to better maintain health and overall well-being."
St. Joseph Hoag Health operates four other wellness centers in Orange County, all of which have been launched within the past year. This includes the DRIVE Wellness Center for Western Digital, which opened in July 2015 at the company's international headquarters in Irvine. The other wellness centers (called Wellness Corners) are also in Irvine at the Jamboree Center business complex, The Village Irvine Spectrum apartments and the Park Place corporate office of St. Joseph Health (the parent organization of St. Joseph Hoag Health).
About St. Joseph Hoag Health
St. Joseph Hoag Health is a historic alliance for Orange County, laying the foundation for sweeping changes in the delivery and accessibility of high quality health care. Combined, St. Joseph Hoag Health has earned some of the highest honors for medical care, including Magnet designation for many of its hospitals (the highest honor bestowed to hospitals for nursing excellence). Drawing upon the strengths of renowned health care organizations, St. Joseph Hoag Health focuses on meeting the big challenges of health care today, including expanding access, improving wellness and preventive services, ensuring quality and developing more efficient methods in the delivery of care. The network includes flagship hospitals (Hoag Newport Beach, Hoag Irvine, Hoag Orthopedic Institute, Mission Hospital Mission Viejo, Mission Hospital Laguna Beach, St. Joseph Hospital Orange and St. Jude Medical Center), eight medical groups and physician networks and numerous outpatient and urgent care facilities in Orange County. The network is also affiliated with CHOC Children's and CHOC Children's at Mission Hospital.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com. @MasimoInnovates
- Media Contact:
Susan Solomon
St. Joseph Hoag Health
Phone: 716-616-4567
Email: susan.solomon@stjoe.orgMedia Contact:
Irene Paigah
Masimo
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Brazil's A.C.Camargo Cancer Center Adopts Masimo SpHb for Noninvasive and Continuous Hemoglobin Monitoring
NEUCHATEL, Switzerland – August 25, 2015 – Masimo (NASDAQ: MASI) announced today that A.C.Camargo Cancer Center in São Paulo, Brazil has installed Masimo's noninvasive and continuous hemoglobin (SpHb®) technology to monitor surgical patients.
Clinicians use invasive blood sampling and laboratory analysis of hemoglobin to determine hemoglobin levels and help estimate blood loss to determine need for red blood cell transfusions. However, lab-based hemoglobin is only available intermittently and results are often delayed. SpHb offers real-time visibility to changes – or lack of changes – in hemoglobin between invasive blood sampling.
"The data associating transfusion with higher mortality rates was clear, so we knew we had to implement strategies to reduce transfusions," said Dr. Eduardo Henrique Joaquim Giroud, anesthesiologist and Director of the Department of Anesthesiology at A.C.Camargo Cancer Center. "This technology enables us to noninvasively and continuously monitor hemoglobin, a strong indicator of blood loss. It provides continuous trend data which, in combination with invasive blood sampling data, helps us determine who actually needs to be transfused and the right amount of blood needed, not more, and not less."
SpHb monitoring may provide additional insight between invasive blood sampling when:
- The SpHb trend is stable and the clinician may otherwise think hemoglobin is decreasing
- The SpHb trend is rising and the clinician may otherwise think hemoglobin is not increasing fast enough
- The SpHb trend is dropping and the clinician may otherwise think hemoglobin is stable
"For over 60 years, the A.C.Camargo Cancer Center has been serving as an international reference center for prevention, multidisciplinary treatment, teaching and research of cancer," stated Joe Kiani, Founder and CEO of Masimo. "We are happy to see them adopt SpHb technology through Masimo's Root® patient monitoring and connectivity platform for the betterment of their patients."
About A.C.Camargo Cancer Center
A.C.Camargo is a private non-profit institution maintained by Antonio Prudente Foundation and is currently one of the largest integrated cancer centers worldwide. With guided activities on four pillars: prevention, treatment, education and cancer research, they serve private patients and the Unified Health System (SUS). In 2014 alone, it had 3.5 million hospital visits with 62% dedicated to patients of the Unified Health System (SUS). It currently has about 40 medical specialties and more than 4000 professionals engaged with the task of 'combating cancer patient to patient' In 2014 alone, A.C.Camargo published over 160 articles.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In the U.S., Masimo rainbow® SET pulse cooximeter instruments and sensors are indicated to provide noninvasive monitoring of arterial oxygen saturation (SpO2) and pulse rate (PR), carboxyhaemoglobin saturation (SpCO®), methaemoglobin saturation (SpMet®), total hemoglobin concentration (g/dl SpHb), and/or respiration rate (RRa®). The devices are not intended to replace laboratory measurements of hemoglobin and have not been cleared by FDA to detect bleeding or reduce the amount or frequency of red blood cell (RBC) transfusions. Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total haemoglobin (SpHb®) contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
- Media Contact:
Irene Paigah
Masimo
Phone: (858) 616-8689
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Reports Second Quarter 2015 Financial Results
Q2 2015 Highlights (compared to Q2 2014):
- Total revenue, including royalties, rose 10.5% to $155.7 million
- Product revenue rose 10.6% to $147.6 million
- Masimo rainbow® revenue rose 16.3% to $13.5 million
- SET® and rainbow® SET® units shipments were a record 44,300
- GAAP net income of $19.4 million, or $0.36 per diluted share versus $13.8 million or $0.24 per diluted share in the year-ago period
- Non-GAAP net income of $23.1 million, or $0.43 per diluted share versus $14.1 million or $0.25 per diluted share in the year-ago period
Irvine, California – August 4, 2015 – Masimo (NASDAQ: MASI)today announced its financial results for the second quarter ended July 4, 2015.
Second quarter 2015 product revenues rose 10.6% to $147.6 million, compared to $133.5 million for the second quarter of fiscal year 2014, and total revenue, including royalties, rose 10.5% to $155.7 million, up from $140.9 million for the second quarter of fiscal year 2014. The unfavorable effect of foreign currency movements reduced second quarter product revenues by approximately $5.5 million.
The company's worldwide direct product revenue in the second quarter of 2015 rose by 10.4% compared to the same period in 2014 and represented 84.7% of product revenue. OEM sales, which accounted for 15.3% of product revenue, rose by 11.5% to $22.5 million in the second quarter of 2015 compared to the same period in 2014. Revenue from sales of Masimo rainbow® products rose by 16.3% to $13.5 million in the second quarter of 2015, compared to the same period in 2014.
GAAP net income for the second quarter of 2015 was $19.4 million, or $0.36 per diluted share, compared to GAAP net income of $13.8 million, or $0.24 per diluted share, in the second quarter of 2014. Non-GAAP net income for the second quarter was $23.1 million, or $0.43 per diluted share, versus non-GAAP net income of $14.1 million or $0.25 per diluted share in the year-ago period. During the second quarter of 2015, the company shipped a record 44,300 SET® pulse oximetry and rainbow® Pulse CO-Oximetry™ units, excluding handheld units. Masimo estimates its worldwide installed base as of July 4, 2015 to be 1,362,000 units, up 8.1% from 1,260,000 units as of June 28, 2014.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "Our second quarter results reflect the continued demand for our SET® Pulse Oximetry technology and our emerging rainbow® SET® Pulse CO-Oximetry technology. The years of building a solid foundation of technologies and products that save and improve lives, along with a build out of our infrastructure to best serve our customers, should allow us to continue to deliver positive returns for our shareholders."
During the six-month period ended July 4, 2015, the company generated $28.3 million in cash from operations and at July 4, 2015, total cash and cash investments were $119.4 million compared to $134.5 million as of January 3, 2015. Also, during the six-month period July 4, 2015, the company repurchased approximately 2.4 million shares of stock for $81.6 million, including 2.1 million shares in the quarter ended July 4, 2015 for $73.4 million.
2015 Financial Guidance
Masimo today is updating its 2015 financial guidance. Masimo now expects fiscal 2015 total revenues to be approximately $621 million, up from $608 million. Total fiscal 2015 product revenues are now expected to be approximately $592 million, up from $580 million and total fiscal 2015 royalty revenues are now projected to be approximately $29 million, up from $28 million. Masimo now also expects its fiscal 2015 GAAP earnings per diluted share to be $1.43, up from $1.33 and expects non-GAAP earnings per diluted share to be approximately $1.61. Masimo will provide additional financial information during the conference call today. Each of the components of Masimo's guidance set forth above is an estimate only and actual performance could differ.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the call will be available online from the investor relations page of the company's website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 84990153. After the live webcast, the call will be available on Masimo's website through September 1, 2015. In addition, a telephonic replay of the call will be available through August 18, 2015. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 84990153.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our expectations for full fiscal year 2015 total and product revenues and GAAP and non-GAAP earnings per diluted share; demand for our products; anticipated revenue and earnings growth; our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies and reduce the cost of care; and demand for our technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET® and Masimo rainbow® SET® products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the amount and type of equity awards that we may grant to employees and service providers in the future; our ongoing litigation and related matters; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
- Investor Contact:
Eli Kammerman
Phone: (949) 297-7077
Email: ekammerman@masimo.com - Media Contact:
Irene Paigah
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation
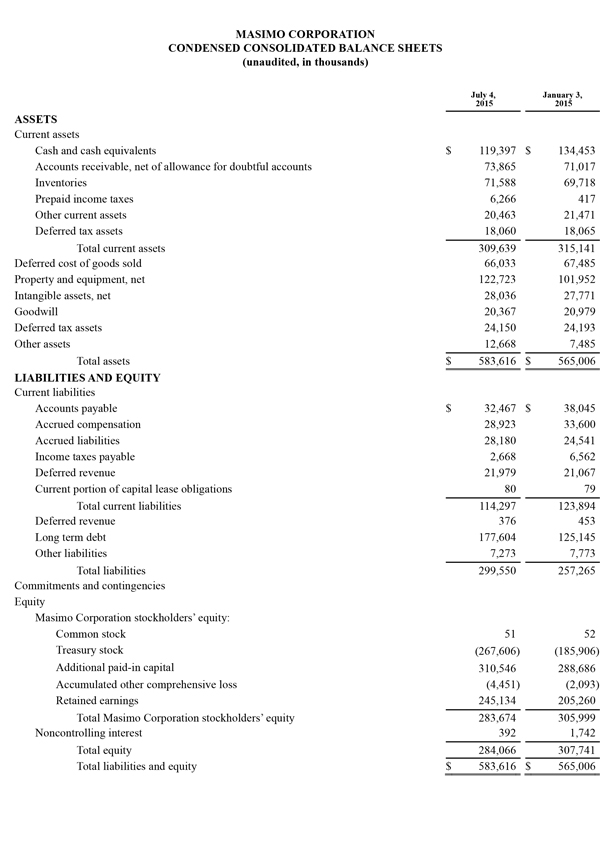
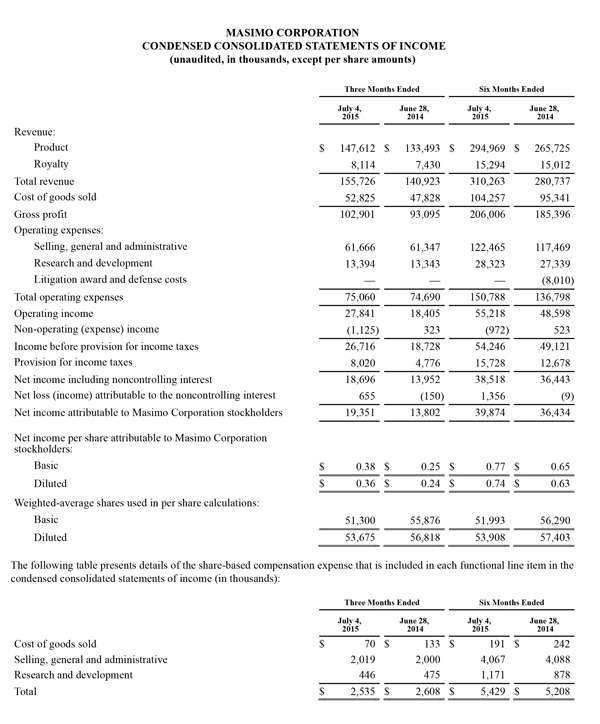
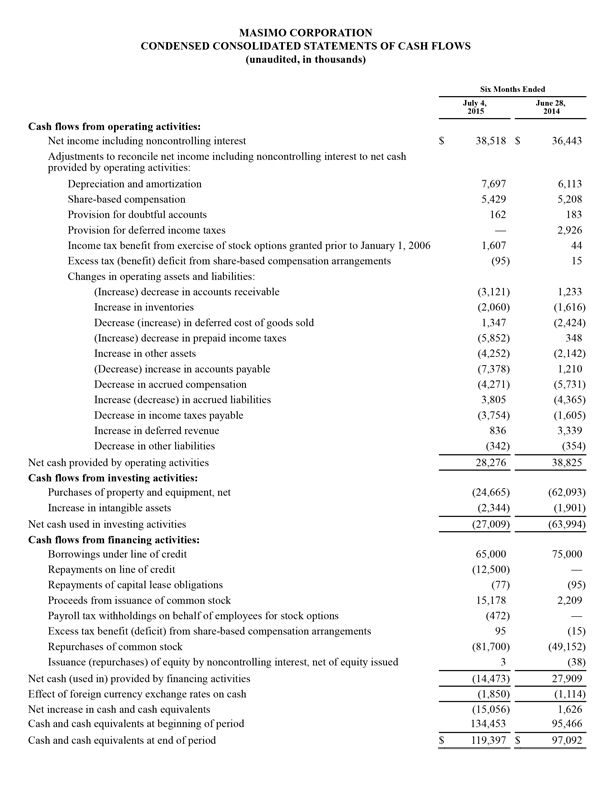
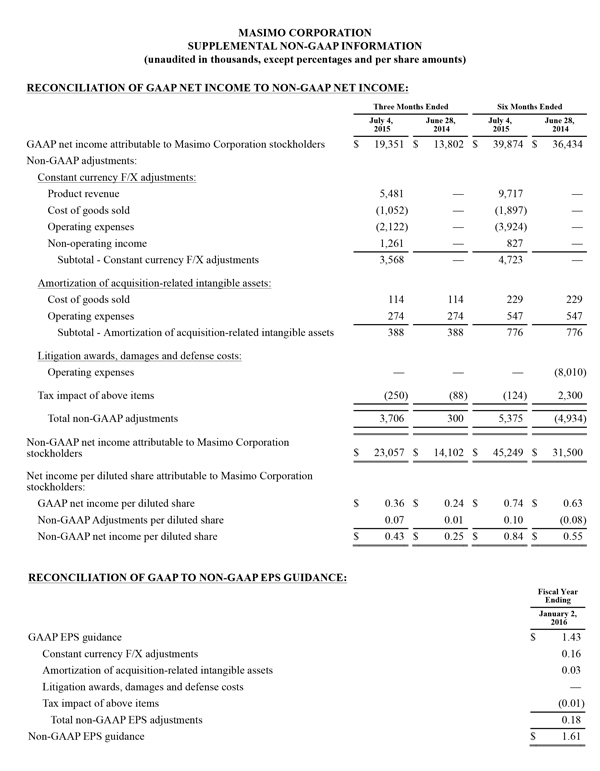
Non-GAAP Financial Measures
The non-GAAP financial measures contained herein are a supplement to the corresponding financial measures prepared in accordance with U.S. generally accepted accounting principles (GAAP). The non-GAAP financial measures presented exclude the items summarized in the above table that are more fully described below. Management believes that adjustments for these items assist investors in making comparisons of period-to-period operating results and that these items are not indicative of the company's ongoing core operating performance. These non-GAAP financial measures have certain limitations in that they do not reflect all of the costs associated with the operations of the company's business as determined in accordance with GAAP. Therefore, investors should consider non-GAAP financial measures in addition to, and not as a substitute for, or as superior to, measures of financial performance prepared in accordance with GAAP. The non-GAAP financial measures presented by the company may be different from the non-GAAP financial measures used by other companies.
The company has presented the following non-GAAP measures on a basis consistent with its historical presentation, to assist investors in understanding the company's core net operating results on an ongoing basis: (i) non-GAAP product revenue, (ii) non-GAAP gross profit, (iii) non-GAAP operating expenses, (iv) non-GAAP non-operating income (expense); (vi) non-GAAP net income attributable to Masimo Corporation stockholders, and (vii) non-GAAP net income per diluted share attributable to Masimo Corporation stockholders. These non-GAAP financial measures may also assist investors in making comparisons of the company's core operating results with those of other companies. Management believes non-GAAP product revenue, non-GAAP gross profit, non-GAAP net income attributable to Masimo Corporation stockholders and non-GAAP net income per diluted share attributable to Masimo Corporation stockholders are important measures in the evaluation of the Company's performance and uses these measures to better understand and evaluate our business.
The non-GAAP financial measures reflect adjustments for the following items, as well as the related income tax effects thereof:
Constant currency F/X adjustments. Some of our sales agreements with foreign customers provide for payment in currencies other than the U.S. Dollar. Similarly, certain of our product costs and operating expenses, and the related balance sheet payables and accruals, are denominated in foreign currencies other than the U.S. Dollar. These foreign currency revenues, costs and expenses, receivables, payables and accruals, when converted into U.S. Dollars, can vary significantly from period to period depending on the average and quarter-end exchange rates during a respective period. We believe that comparing these foreign currency denominated revenues, costs and expenses, receivables, payables and accruals by holding the exchange rates constant with the prior year period is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. We anticipate that fluctuations in foreign exchange rates and these related constant currency and other foreign exchange adjustments will continue to occur in future periods.
Amortization of acquisition-related intangibles. Amortization of intangibles generally represents costs incurred by an acquired company or other third party to build value prior to our acquisition of the intangible assets. As such, it is effectively part of the transaction costs of the acquisition rather than ongoing costs of operating our core business. As a result, we believe that exclusion of these costs in presenting non-GAAP financial measures provides management and investors a more effective means of evaluating its historical performance and projected costs and the potential for realizing cost efficiencies within our core business. Amortization of intangibles will recur in future periods.
Litigation awards, damages and defense costs. In connection with litigation proceedings arising in the course of our business, we have previously recorded expenses as a defendant in such proceedings in the form of damages and directly-related legal fees, as well as reversals of such damages and directly-related legal fee expenses upon a court vacating a prior award against us. In addition, we have also previously recorded recoveries of damages and directly-related legal fees as a plaintiff in litigation proceedings. We believe that exclusion of these expenses, expense reversals and recoveries is useful to management and investors in evaluating the performance of our ongoing operations on a period-to-period basis. In this regard, we note that these expenses, expense reversals and recoveries are generally unrelated to our core business and/or infrequent in nature. Lease termination exit costs. The effects of lease termination exits costs are excluded resulting from the purchase of the New Hampshire manufacturing facility that Masimo previous leased.
Lease termination exit costs. The effects of lease termination exits costs are excluded resulting from the purchase of the New Hampshire manufacturing facility that Masimo previous leased.
Reconciliations between the GAAP and non-GAAP amounts for each financial statement line item are as follows:
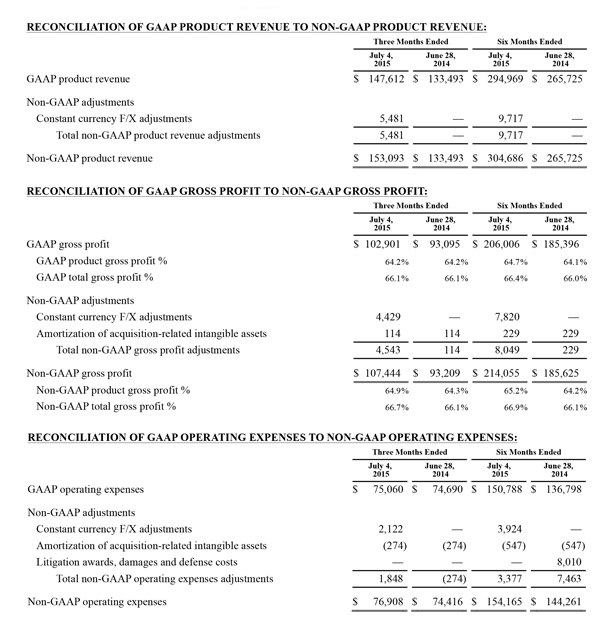
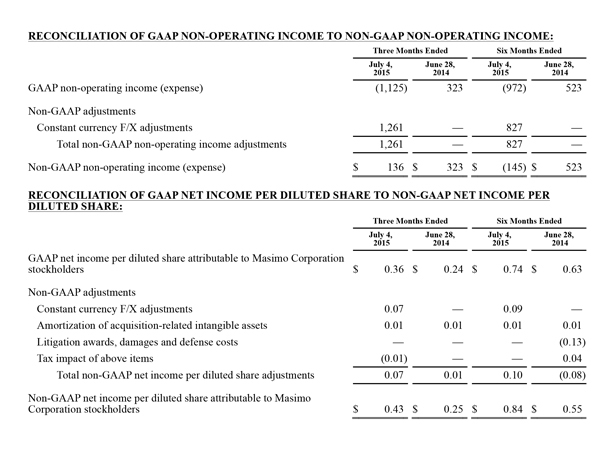


Masimo Renews its $1,000,000 Performance Guarantee that Masimo SET® Technology will Outperform All Nellcor™ Pulse Oximeters
Addresses the Inaccurate and Misleading Claims that Covidien Continues to Make Regarding Nellcor Pulse Oximetry Performance
Irvine, California – July 13, 2015 – Masimo (NASDAQ: MASI) the inventor of measure-through motion and low perfusion pulse oximetry, today renewed its $1,000,000 (USD) performance guarantee that Masimo SET® Pulse Oximetry will outperform all Nellcor (part of Covidien and now Medtronic) pulse oximeters. Under the guarantee, if Masimo SET is proven not to be superior to all Nellcor pulse oximeters, including the three that Covidien announced have received FDA 510(k) clearance with motion claims, Masimo will pay up to $1,000,000 to help a hospital acquire that pulse oximetry.
"As a medical technology company, we believe it is our obligation to be truthful and open in our advertising because ultimately patient care is at issue. We continue to be perplexed by the misleading marketing tactics of our competitor," stated Joe Kiani, Founder and CEO of Masimo. "We are concerned that customers may be misled and we hope our guarantee will cause them to question what they are being told and evaluate the performance of the technologies for themselves."
In addition to renewing the $1 million guarantee, Masimo has updated its website to respond to each of the inaccurate and misleading claims in detail: https://www.masimo.com/nellcorfiction/
Masimo makes four promises to every hospital that to date, Covidien has not been willing to make:
We will demonstrate the superior pulse oximetry performance of Masimo SET® over Nellcor in a side-by-side comparison.
We will provide independent and objective scientific evidence of Masimo SET® superiority over Nellcor, as well as the evidence demonstrating Masimo SET® helps clinicians improve clinical and financial outcomes.
We strongly encourage scientific side-by-side evaluations of Masimo SET® and Nellcor pulse oximetry in challenging conditions with automated data collection.
We are so confident that an objective comparison will reveal the clinical superiority of Masimo SET®, we continue to offer a $1,000,000 performance guarantee.
Please note: This offer is available only to hospitals whose goal is to upgrade their pulse oximetry hospital-wide to the new standard. Important details, conditions, and qualifications for the challenge are available at http://www.masimo.com/1millionrules.htm.
@MasimoInnovates || #Masimo
- Eipe N et al. Indian Journal of Anesthesia. 2006; 50(1): 35-38.
- Ram GG et al. Chin J Traumatol. 2014;17(4):225-8.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors related to MightySat, including our belief in the breakthrough ability of Masimo SET® pulse oximetry to measure-through motion and low perfusion; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Irene Paigah
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Turkey's Leading University Hospital Adopts Masimo SpHb for Continuous Noninvasive Haemoglobin Monitoring
NEUCHATEL, Switzerland – June 30, 2015 – Masimo (NASDAQ: MASI) announced today that Turgut Ozal Medical Centre Liver Hospital in Malatya, Turkey has installed Masimo's noninvasive, continuous haemoglobin (SpHb®) technology to monitor surgical patients.
SpHb is a breakthrough measurement that noninvasively and continuously measures total haemoglobin in the blood, and offers real-time visibility to changes – or lack of changes – in haemoglobin between invasive blood sampling. Laboratory haemoglobin values are only available intermittently and are often delayed. Clinicians can estimate blood loss, but this can both overstate and understate actual blood loss.1,2
"In many cases with severe bleeding during liver transplantation, we had to do the blood transfusion on the patient without having the opportunity to send a sample or the time required to obtain the results," said Professor Dr. Hüseyin Ilksen Toprak, anaesthesiologist at Turgut Ozal Medical Centre. "Sometimes, the amount of blood transfused exceeded that required by the patient. However, today, thanks to routine SpHb monitoring, I am able to immediately visualize both the level of bleeding and the therapeutic effect."
SpHb monitoring may provide additional insight between invasive blood sampling when:
-
The SpHb trend is stable and the clinician may otherwise think haemoglobin is dropping
-
The SpHb trend is rising and the clinician may otherwise think haemoglobin is not rising fast enough
-
The SpHb trend is dropping and the clinician may otherwise think haemoglobin is stable
"In the area of liver transplantation, Turgut Ozal Medical Centre is recognized as one of the top five hospitals in the world," stated Joe Kiani, Founder and CEO of Masimo. "Their hospital was designed to be one of the most modern in the country. They had already been using our SET® Measure-Through Motion and Low Perfusion pulse oximetry. We are happy to see them adopt SpHb technology through Masimo's Root® patient monitoring and connectivity platform."
The Turgut Ozal Medical Centre is in the campus of the Inonu University. The hospital has 16 floors, 1024 beds, 31 services, 12 intensive care units and 26 operating rooms. In addition to liver transplants, the medical centre also handles kidney transplants, bone marrow transplants and burn injuries.
In the U.S., Masimo rainbow® SET pulse co-oximeter instruments and sensors are indicated to provide noninvasive monitoring of arterial oxygen saturation (SpO2) and pulse rate (PR), carboxyhaemoglobin saturation (SpCO®), methaemoglobin saturation (SpMet®), total Haemoglobin concentration (g/dl SpHb), and/or respiration rate (RRa®). SpHb monitoring is intended to supplement, not replace, laboratory measurements. Blood samples should be analysed by laboratory instruments when possible prior to clinical decision-making.
@MasimoInnovates || #Masimo
- Eipe N et al. Indian Journal of Anesthesia. 2006; 50(1): 35-38.
- Ram GG et al. Chin J Traumatol. 2014;17(4):225-8.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total haemoglobin (SpHb®), oxygen content (SpOC™), carboxyhaemoglobin (SpCO®), methaemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total haemoglobin (SpHb®) contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Irene Paigah
Phone: (858) 859-7001
Email: irenep@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Receives CE Mark for MightySat Rx Pulse Oximeter
World's First Wireless Fingertip Pulse Oximeter with Masimo SET Technology Enables Clinicians to Track and Trend Measurements through Smart Mobile Devices
NEUCHATEL, Switzerland – June 22, 2015 – Masimo (NASDAQ: MASI) today announced the CE Mark and full market release of MightySat™ Rx fingertip pulse oximeter for clinical use in CE countries, offering the same Masimo SET® Measure-Through Motion and Low Perfusion™ pulse oximetry technology used in the world's leading hospitals.
Bluetooth®-enabled versions of MightySat Rx allow clinicians to track and trend up to 12 hours of patient measurements on smart mobile devices, and to share that data via standard CSV files as well as transfer to Apple's Health app.
MightySat™ Rx fingertip pulse oximeter provides oxygen saturation (SpO2), pulse rate (PR), Perfusion Index (PI), and Pleth Variability Index (PVI®).
MightySat Rx enables accurate noninvasive spot-checking of oxygen saturation (SpO2) and pulse rate (PR) for adult and paediatric patients even during motion and low perfusion. MightySat Rx measures and displays Perfusion Index (PI), which reflects the strength of the pulsatile signal. MightySat can also measure and display Pleth Variability Index (PVI®), a measure of the dynamic changes in the perfusion index that occur during one or more complete respiratory cycle/s.
"Many clinicians around the world rely on pulse oximetry as the fifth vital sign," stated Joe Kiani, Founder & CEO of Masimo. "The MightySat Rx represents a significant milestone. To deliver Masimo SET performance in this low-power form factor took some engineering, given the precision of our technology and SET's computationally intensive algorithm. We actually dropped the power consumption by nearly two orders of magnitude. But the development effort was worth it, as we expect this technology will empower clinicians and they will no longer have to compromise performance for mobility."
MightySat Rx is available in three versions for patients who weigh more than 30 kg (66 lbs):
-
MightySat Rx – Measures and displays SpO2, PR, and PI
-
MightySat Rx, Bluetooth – Adds Bluetooth LE radio for transfer of parameter data to Apple iOS® and select Android™ mobile devices
-
MightySat Rx, Bluetooth and PVI
Standard features include:
-
Measure-Through Motion and Low Perfusion performance
-
Signal I.Q.® to assess measurement confidence
-
Rugged, lightweight design for operation in challenging environments
-
Long battery life–up to 15 hours with two standard AAA alkaline batteries
-
Comfortable design with silicon finger pad to mold to patients' fingers
Bluetooth is a registered trademark owned by Bluetooth SIG, Inc. Apple is a registered trademarks of Apple Inc. registered in the U.S. and other countries. IOS is a trademark or registered trademark of Cisco in the U.S. and other countries and is used under license. Android is a trademark of Google Inc. MightySat Rx is currently unavailable in the U.S.
@MasimoInnovates || #Masimo
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors related to MightySat, including our belief in the breakthrough ability of Masimo SET® pulse oximetry to measure-through motion and low perfusion; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo iSpO2 Pulse Oximeter Set to Measure Audience Reaction to "Personal Gold" Documentary Celebrating U.S. Olympic Silver Medalists
Quantified Self Conference + Expo in San Francisco Offers Ideal Setting for
Social Experiment
IRVINE, California – June 18, 2015 – Masimo (NASDAQ: MASI), maker of breakthrough Measure-through Motion and Low Perfusion™ pulse oximetry, announced today that for the first time moviegoers attending a screening of the award-winning documentary "Personal Gold: An Underdog Story" will use Masimo's iSpO2® pulse oximeter to help review the film.
MightySat™ fingertip pulse oximeter provides oxygen saturation (SpO2), pulse rate (PR), perfusion index (PI), and Pleth Variability Index (PVI®).
Select attendees of the "Personal Gold" screening at the QS15 Quantified Self Conference + Expo this weekend will receive an iSpO2 to track their blood oxygenation and pulse rate during the film. The Personal Gold team will overlay, graph and post the measurements on a timeline showing physiological reactions to the movie.
The film features the consumer version of Masimo's iSpO2 pulse oximeter. Masimo has since introduced the MightySat fingertip pulse oximeter with optional Bluetooth capability, enabling consumers to track and trend oxygenation and pulse rate measurements on their iOS or select Android mobile devices.
Heralded as "Miracle on Ice-meets-Moneyball" by Wall Street Journal reporter Reed Albergotti, "Personal Gold" gives a behind-the-scenes experience of four underdog women cyclists who become America's hope for a medal at the 2012 London Olympics after the men's team was banned during the Lance Armstrong drug scandal. The underfunded women relied on pulse oximetry and other technologies as part of an innovative "Data not Doping" training regimen to win the first U.S. Women's Track Cycling medal in more than 20 years.

"We believe this is the first time a pulse oximeter has been used to help 'review' a movie," said Joe Kiani, Founder and CEO of Masimo. "Masimo is dedicated to taking noninvasive monitoring to new sites and applications. The Quantified Self Expo offers an ideal setting to noninvasively measure physiological changes of viewers as they watch this inspiring film."
"'Personal Gold' is more than a must-see Olympic story – it's a film that expertly explores the intersection of sports, science and ingenuity," said Gary Wolf, contributing editor at Wired and host of this weekend's screening. "Without access to the tools of self-measurement, such as the noninvasive pulse oximetry device we'll be using at the screening this weekend, these heroic athletes would not have had a chance to make history. For these women, Quantified Self techniques meant the difference between standing on the side lines and standing atop a podium."
What: Personal Gold: An Underdog Story - Exclusive Event @ Quantified Self Expo
Hosted by Gary Wolf, contributing editor at Wired
Where: 2015 Quantified Self Expo in the newly renovated 400-seat Cowell Theater, Fort Mason Center, San Francisco, CA 94123
When: Saturday, June 20, 2015. 2:30 pm doors open; 3 pm sharp screening begins; 4:30 pm Q&A with Olympic medalist and filmmakers
https://vimeo.com/ondemand/prsnlgldndrdgstr
@MasimoInnovates
@quantifiedself
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion™ pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors related to MightySat, including our belief in the breakthrough ability of Masimo SET® pulse oximetry to measure-through motion and low perfusion; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces Root CE Marking with Blood Pressure and Temperature
NEUCHATEL, Switzerland – June 15, 2015 – Masimo (NASDAQ: MASI) today announced the CE Mark and pre-market release of Root® connectivity and patient monitoring platform with noninvasive blood pressure and temperature capabilities.

Root with noninvasive blood pressure from SunTech Medical enables clinicians to measure arterial blood pressure for adult, pediatric, and neonatal patients, with three distinct measurement modes: spot-check, automatic interval, and stat interval.
The temperature module from Welch Allyn is indicated to measure temperature of adult, pediatric and neonatal patients.
"Root and Patient SafetyNet powered with Masimo SET has changed for the better our patient care and patient safety," said Caroline Stade, Chief Nursing Officer at University Children's Hospital Basel (UKBB) in Basel, Switzerland. "With remote alarm notification from Patient SafetyNet, we've been able to give proper care to patients in need without the false alarms that made proper care previously untenable. With the addition of blood pressure monitoring, we can provide patients in sub-acute care an integrated solution that will provide more efficient and effective care for our patients. We all, the physicians and nurses, are very happy with this new system and would never ever disclaim or work without the Root monitor."
Root offers a high visibility display with intuitive, touchscreen navigation for easy and adaptable use in any hospital environment:
- Integrates measurements from Masimo Radical-7® handheld for SET® Measure-through Motion and Low Perfusion™ pulse oximetry, continuous haemoglobin (SpHb®), PVI®, ORI™, and other noninvasive rainbow® parameters, or the Radius-7™ patient-worn monitor for continuous monitoring of mobile patients
- Flexible measurement expansion through Masimo Open Connect™ (MOC-9™), including:
- ISA™ CO2 capnography for sidestream monitoring featuring Nomoline™ technology for extended monitoring and cost-effective consumables
- O3™ regional oximetry for the simultaneous measurement of regional oxygen saturation (rSO2) and arterial oxygen saturation (SpO2) to help clinicians detect regional hypoxemia that pulse oximetry alone can miss, and
- SedLine® brain function monitoring, which provides four channels of EEG to help clinicians better understand the effects of anaesthesia on the brain. SedLine measures the effects of anaesthesia and sedation by monitoring both sides of the brain's electrical activity.
- Automatic display of parameters, waveforms, and viewing configuration based on the clinician's presence through MyView™ technology enabled with Patient SafetyNet™ version 5000 or higher
- Enables clinicians to easily and automatically document vital signs data, measured from any connected device or manually entered, directly from Root to the patient's Electronic Medical Record (EMR). Alarms and alerts for all devices are seamlessly forwarded to the patient's clinician and all device data are effortlessly documented in the patient's EMR.
Root with noninvasive blood pressure and temperature is currently not available in the U.S.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total haemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that Masimo's Root Vital Signs Monitoring is adaptable to any hospital environment; risks related to our assumptions regarding the repeatability of clinical results; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Root Wins the GOLD 2015 Medical Design Excellence Award
IRVINE, California – June 10, 2015 – Masimo (NASDAQ: MASI) announced that its Root® patient monitoring and connectivity platform has received the GOLD 2015 Medical Design Excellence Award—the highest award given in the general hospital devices and therapeutic products category. The 2015 Medical Design Excellence Award winners were honored today at a presentation ceremony in New York City's Jacob K. Javits Convention Center, in conjunction with the Medical Design & Manufacturing (MD&M) East Conference and Exposition (https://www.advancedmanufacturingeast.com/en/show-brands/mdm-east.html).
Root comes with a dock for the Radical-7® handheld monitor or Radius-7® wearable monitor, an intuitive display, and multiple networking/connectivity options. Root integrates multiple streams of data on an easy-to-read, touch-screen.
Root innovations include:
- MOC-9™ – Flexible measurement expansion through Masimo Open Connect™ (MOC-9™) with MOC-9 modules from Masimo or third-party measurement by other companies to expand the platform's measurements and capabilities. New MOC-9 modules in the U.S. will require new 510(k) clearances
- ISA™ Capnography – CO2 sidestream module featuring fast warm-up time and the innovative and cost-effective Nomoline™ sampling line
- SedLine® brain function monitoring module, which provides four channels of EEG to help clinicians better understand the effects of anesthesia on the brain
- Iris™ connectivity gateway designed to provide integration for standalone devices such as IV pumps, ventilators, hospital beds, and other patient monitors. Iris enables Root to intake data from all devices connected to the patient, acting as an in-room patient monitor and connectivity hub. Alarms and alerts for all devices are seamlessly forwarded to the patient's clinician and all device data are effortlessly documented in the patient's electronic medical record
- Wireless functionality – Capable of transmitting information through Bluetooth and Wi-Fi.

Root can accommodate Radical-7 or Raidus-7, the first and only wearable, wireless monitor with Masimo's breakthrough rainbow® SET® technology, to measure blood oxygenation, pulse rate, and rainbow® acoustic monitoring (RAM™) through acoustic respiration rate (RRa®). Radius-7 offers patients continuous monitoring while providing freedom of movement, allowing hospitals to optimize capacity by getting patients out of bed faster. Studies have shown that patient mobility is a key factor in more rapid patient recovery.1
The Medical Design Excellence Awards competition (https://www.advancedmanufacturingeast.com/en/show-brands/mdm-east.html) is organized and presented by UBM Canon and is the only awards program that exclusively recognizes contributions and advances in the design of medical products. Entries are evaluated on the basis of their design and engineering features, including innovative use of materials, user-related functions that improve healthcare delivery and change traditional medical attitudes or practices, features that provide enhanced benefits to the patient, and the ability of the product development team to overcome design and engineering challenges so that the product meets its clinical objectives. A comprehensive review of the entries was performed by an impartial, multidisciplinary panel of third-party jurors with expertise in biomedical engineering, human factors, industrial design, medicine, and diagnostics.
"We are honored to have Root receive the GOLD MDEA-winning product," said Joe Kiani, Founder and CEO of Masimo. "We hope Root will eliminate barriers to entry for innovative products and barriers to interoperability and help automate patient care with its open and scalable architecture."
1 Needham D, Korupolu R, Zanni J, Pradhan P, Colantuoni E, Palmer J, Brower R, Fan E. "Early Physical Medicine and Rehabilitation for Patients With Acute Respiratory Failure: A Quality Improvement Project." Archives of Physical Medicine and Rehabilitation Vol 91, Issue 4, PP 536–542, April 2010
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


New Study Presented at the 2015 Euroanaesthesia Congress Evaluated the Impact on Mortality and Morbidity with Masimo SpHb and PVI
NEUCHATEL, Switzerland, June 5, 2015 – Masimo (NASDAQ: MASI) announced today a new clinical study presented at the European Society of Anaesthesiology's Euroanaesthesia 2015 Annual Congress in Berlin, Germany. The study evaluated Masimo's parameters of noninvasive, continuous haemoglobin, SpHb®, and fluid responsiveness, PVI®, with patients in hospital settings.*
Dr. Sebastien Ponsonnard and researchers at CHU Limoges, Department of Anaesthesiology & Intensive Care, in Limoges, France, evaluated the impact on mortality and morbidity in patients who underwent general anaesthesia at the hospital after the introduction of Masimo SpHb continuous haemoglobin measurement and response to fluid loading by PVI.1
Operating rooms and ICUs were equipped with Masimo Radical-7 Pulse CO-Oximeter® monitors. Over a six-month period (Feb. 6-Aug. 7, 2014), patients receiving general anaesthesia were monitored noninvasively.
At one month, mortality decreased in 2014 (vs. 84/5123 = 1.64% vs. 121/5478 = 2.2%, P = 0.024). In-hospital mortality was not different between the two years. Death in cardiothoracic surgery was slightly lower (P = 0.07).
Researchers concluded: "These results suggest that by using a non-invasive monitor, measuring SpHb and fluid loading responsiveness is possible on a large scale. The observed reduction of mortality agrees with multi-centric randomized studies using more invasive monitoring systems2 and support the large use of such a device."
* For CE Marking:
- The Radical-7 is intended for the continuous and noninvasive monitoring of SpO2, SpCO, SpMet and SpHb.
- The Radical-7 with PVI has been clinically evaluated for fluid responsiveness measurements.
For the US market:
- The Radical-7 is indicated for the continuous and noninvasive monitoring of SpO2, SpCO, SpMet and SpHb.
- The Radical-7 with PVI is a measure of dynamic changes in the perfusion index (PI) that occur during the respiratory cycle. The PVI calculation is accomplished by measuring changes in PI over a time interval where one or more complete respiratory cycles have occurred. PVI is displayed as a percent (0-100%). PVI may show changes that reflect physiologic factors such as vascular tone, circulating blood volume and intrathoracic pressure excursions. The utility of PVI is unknown at this time and requires further clinical studies. Technical factors that may affect PVI include probe malposition and patient motion.
- Ponsonnard S, Yonnet S, Marin B, Cros J, Ben Miled S, Nathan N. "Continuous Hb and plethysmography variability index (PVI) monitoring is associated to a decreased mortality at the scale of a whole hospital." Proceedings of the European Society of Anaesthesiology's Euroanaesthesia 2015 Annual Congress, May 30-June 2, Berlin, Germany, 16AP3-2, Room A1 – Poster Abstract Presentation Session, e-Board 8
- Hamilton et al. AnesthAnalg. 2011
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com. @masimoinnovates
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Court Upholds Award of $467 Million to Masimo for Philips' Patent Infringement
Irvine, California – May 19, 2015 – Masimo (NASDAQ: MASI), inventor of breakthrough noninvasive Measure-through Motion and Low Perfusion™ pulse oximetry and rainbow® Pulse CO-Oximetry™, announced today that the Delaware federal court upheld a jury verdict of $467 million against Philips Electronics North American Corporation and Philips Medizin Systeme Boblingen GMBH for infringing two Masimo patents.
In so doing, the District Court rejected all of Philips' numerous invalidity challenges, denied Philips' motion for a new trial, denied Philips' motion to lower the damages award, and held that Masimo did not commit inequitable conduct in obtaining one of the infringed patents. Because the District Court also determined that Philips had pled a misuse defense, which had been stayed earlier in the case, the court asked that the parties submit proposals on how to proceed.
After years of unsuccessfully trying to reach an out-of-court solution to Philips' patent infringement of Masimo's breakthrough Measure-through Motion and Low Perfusion pulse oximetry technology, on Feb. 3, 2009, Masimo filed its patent infringement action in Delaware. Philips asserted, among other things, that it did not infringe Masimo's patents, although it later conceded infringement, and then argued Masimo's patents were invalid. After a 10-day trial, a jury on Oct. 1, 2014 returned a verdict in favor of Masimo on every issue and awarded Masimo $466,774,783 in damages.
Joe Kiani, founder and CEO of Masimo said, "This ruling reaffirms Masimo's innovative creation of measure-through-motion pulse oximetry that has dramatically improved patient care worldwide."
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion™ pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®) in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors discussed in the Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Perioperative Enhanced Recovery Program that Included Masimo PVI Led to Significant Reductions in Length of Stay, Complications and Costs
Neuchatel, Switzerland – May 14, 2015 – Masimo (NASDAQ: MASI) announced today a study published in the Journal of the American College of Surgeons found that implementing a multidisciplinary perioperative Enhanced Recovery protocol that included intraoperative fluid management guided by a goal-directed algorithm using Masimo PVI® led to significant reductions in length of stay (LOS), complication rates, and cost for patients undergoing both open and laparoscopic colorectal surgery.1Masimo's noninvasive PVI is a measure of the dynamic changes in the perfusion index that occur during one or more complete respiratory cycles.
In the retrospective study of the Enhanced Recovery program implemented at the University of Virginia (UVA), Dr. Robert Thiele and colleagues compared the results of 109 patients managed with the program to 98 consecutive patients before the program was implemented. The Enhanced Recovery program included ingestion of a carbohydrate drink two hours prior to surgery, pre-operative multimodal analgesic regimen, goal-directed therapy with Masimo's PVI, intraoperative low-dose spinal morphine, limiting intraoperative opiates, intraoperative infusions of ketamine and lidocaine (continued 48 hours post-operatively), early mobilization, and oral intake post-operatively.
Researchers observed that, as compared to the standard of care group, patients in the Enhanced Recovery program had:
- 2.3 fewer days of hospitalization (4.6 ± 3.6 vs 6.8 ± 4.7, p = 0.0002)
- Reduced mean direct cost ($13,306 vs $20,435, equating to savings of $777,061, p < 0.001)
- Fewer surgical site infections (7.3% vs 20.4%, p = 0.008)
- Less fluid administered (848 mL vs 2,733 mL, p < 0.0001)
- Lower total hospital morphine equivalents (63.7 mg vs 281 mg, p < 0.0001)
- Considerable improvements in patient satisfaction scores
- Overall satisfaction increased to the 59th percentile, from 26th
- "Felt ready for discharge" increased to the 99th percentile, from 41st
- Satisfaction with pain control increased to the 98th percentile, from 43rd
- Likelihood that patients would recommend the hospital increased to the 89th percentile, from 32nd
Researchers stated: "The 2.3-day reduction in LOS for the 109 patients on the ER protocol equated to a savings of 261 patient-bed days. Given that the UVA institutional average LOS is 5.5 days, this allowed the Medical Center to admit 47.5 additional patients during this time period as the direct result of the protocol."
The study concluded: "Implementation of an ER protocol led to improved patient satisfaction and significant reduction in LOS, complication rates, and cost for patients undergoing both open and laparoscopic colorectal surgery. These data demonstrate that small investments in the perioperative environment can lead to large returns."
1 Thiele RH, Rea KM, Turrentine FE, Friel CM, Hassinger TE, Goudreau BJ, Umapathi BA, Kron IL, Sawyer RG, Hedrick TL, Standardization of Care: Impact of an Enhanced Recovery Protocol on Length of Stay, Complications, and Direct Costs after Colorectal Surgery, Journal of the American College of Surgeons (2015), doi: 10.1016/j.jamcollsurg.2014.12.042.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com. @MasimoInnovates
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using Masimo PVI, risks related to our belief that PVI is an easy-to-use and cost-effective measure for assessing whether patients will benefit from fluid administration, risks related to our assumptions that PVI enables personalized and goal-directed fluid therapy, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Joe Kiani Launches Social Media Presence
Irvine, California – May 7, 2015 – Masimo Founder and CEO Joe Kiani has started tweeting! Follow @JoeKiani on Twitter for his latest updates.
Not on Twitter? It's easy to set up. Just follow these instructions: https://help.twitter.com/en/resources/twitter-guide/twitter-101/how-to-set-up-your-twitter-account-twitter-help

This year, Masimo ramped up its social media presence, beginning with the rollout of a highly successful, real-time picture contest at CES 2015. Attendees who took pictures with Olympic silver medalist Dotsie Bausch or Guinness World Record holder Stig Severinsen and used the proper hashtags had a chance to win a MightySat.
Follow Masimo on Twitter through @MasimoInnovates for the latest company updates, news and event information.
The Patient Safety Movement Foundation has built social momentum for the Patient Safety, Science & Technology Summit with the introduction of a mobile app that allowed attendees and others from around the world to post comments and even ask questions of panelists in real-time.
To get the latest updates on the Patient Safety Movement, be sure to follow @PLAN4ZERO as the Patient Safety Movement moves toward accomplishing its goal of eliminating preventable patient deaths by 2020!
As Masimo and the Patient Safety Movement Foundation evolve their social media presence, we're eager to hear your feedback, please email: CorporateCommunications@masimo.com with your ideas and suggestions.
#Masimo || #0X2020
Media Contacts:
Mike Drummond
Masimo
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademark of Masimo.


Masimo Reports First Quarter 2015 Financial Results
Q1 2015 Highlights (compared to Q1 2014):
- Total revenue, including royalties, rose 11% to $154.5 million
- Product revenue rose 11% to $147.4 million
- Masimo rainbow® revenue declined 5% to $12.2 million
- SET® and rainbow® SET® units shipments were 44,000
- Net income of $20.5 million, or $0.38 per diluted share versus $22.6 million or $0.39 per diluted share in the year-ago period; year-ago period earnings per diluted share included a $0.09 benefit from legal accrual reversal
- Non-GAAP net income of $22.2 million, or $0.41 per diluted share versus $17.9 million or $0.31 per diluted share in the year-ago period
Irvine, California, May 6, 2015 – Masimo (NASDAQ: MASI) today announced its financial results for the first quarter ended April 4, 2015.
First quarter 2015 product revenues rose 11% to $147.4 million, compared to $132.2 million for the first quarter of fiscal year 2014, and total revenue, including royalties, rose 11% to $154.5 million, up from $139.8 million for the first quarter of fiscal year 2014. The unfavorable effect of foreign currency movements reduced first quarter product revenues by approximately $4.2 million.
The company's worldwide direct product revenue in the first quarter of 2015 rose by 13% compared to the same period in 2014 and represented 85% of product revenue. OEM sales, which accounted for 15% of product revenue, rose by 6% to $22.3 million in the first quarter of 2015 compared to the same period in 2014. Revenue from sales of Masimo rainbow® products was $12.2 million in the first quarter of 2015, compared to $12.9 million in the prior year period.
GAAP net income for the first quarter of 2015 was $20.5 million, or $0.38 per diluted share, compared to GAAP net income of $22.6 million, or $0.39 per diluted share, in the first quarter of 2014. First quarter 2014 results were positively impacted by a $0.09 per diluted share benefit due to the impact of a legal expense reversal. Non-GAAP net income for the first quarter was $22.2 million, or $0.41 per diluted share, versus non-GAAP net income of $17.9 million or $0.31 per diluted share in the year-ago period. During the first quarter of 2015, the company shipped approximately 44,000 SET® pulse oximetry and rainbow® Pulse CO-Oximetry™ units, excluding handheld units. Masimo estimates its worldwide installed base as of April 4, 2015 to be 1,340,000 units, up 9% from 1,231,000 units as of March 29, 2014.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "Our first quarter results continue to illustrate the strength of our pulse oximetry and Pulse CO-Oximetry™ technology, as we shipped over 40,000 Root®, Radical-7®, Radius-7TM and Rad-8® monitors and MS and MX OEM boards in the period. We are increasing our full year financial guidance for both revenues and earnings per share as a result of our strong first quarter performance, and we remain encouraged in our outlook for the remainder of the year."
During the first quarter, the company generated $19.1 million in cash from operations and used approximately $13.1 million to fund improvements to its corporate headquarters facility. In addition, the company repurchased approximately 0.3 million shares of stock for $8.2 million, of which $2.1 million settled during the quarter. As of April 4, 2015, Masimo's cash and cash equivalents were $135.7 million, compared to $134.5 million as of January 3, 2015.
2015 Financial Guidance
Masimo today is updating its 2015 financial guidance. Masimo now expects fiscal 2015 total revenues to be $608 million, up from $605 million and total product revenues to be $580 million, up from $577 million. Masimo now also expects its GAAP earnings per diluted share to be $1.33, up from $1.30 and expects non-GAAP earnings per diluted share to be approximately $1.48. Masimo will provide additional financial information during the conference call today. Each of the components of Masimo's guidance set forth above is an estimate only and actual performance could differ.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the call will be available online from the investor relations page of the company's website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 32778491. After the live webcast, the call will be available on Masimo's website through May 20, 2015. In addition, a telephonic replay of the call will be available through May 20, 2015. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 32778491.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our expectations for full fiscal year 2015 total and product revenues and GAAP and non-GAAP earnings per diluted share; statements regarding product standard product margins, expense controls, our market share and adoption of our newer products; our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies and reduce the cost of care; and demand for our technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET® and Masimo rainbow® SET® products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the amount and type of equity awards that we may grant to employees and service providers in the future; our ongoing litigation and related matters; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
| Investor Contact Eli Kammerman Masimo Phone: (949) 297-7077 Email: ekammerman@masimo.com | Media Contacts: Mike Drummond Masimo Phone: (949) 297-7434 Email: mdrummond@masimo.com |
|---|
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation
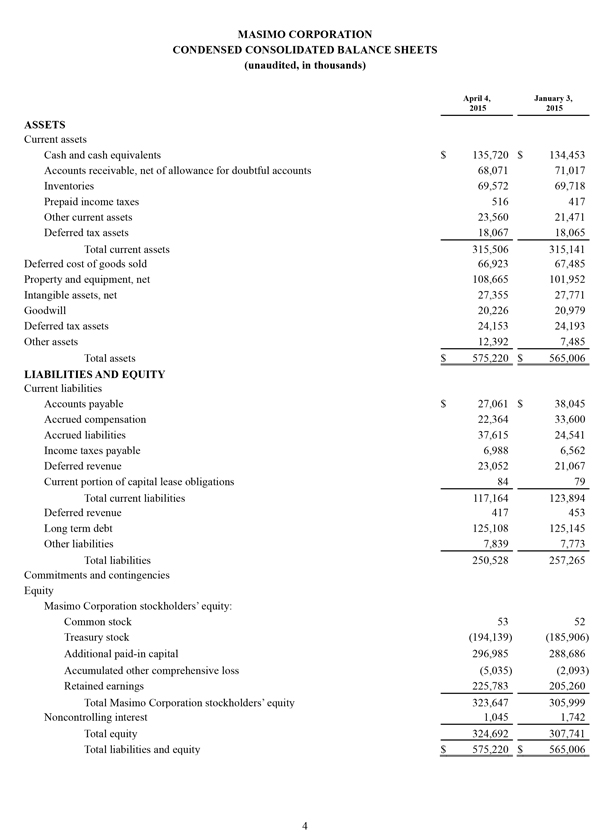
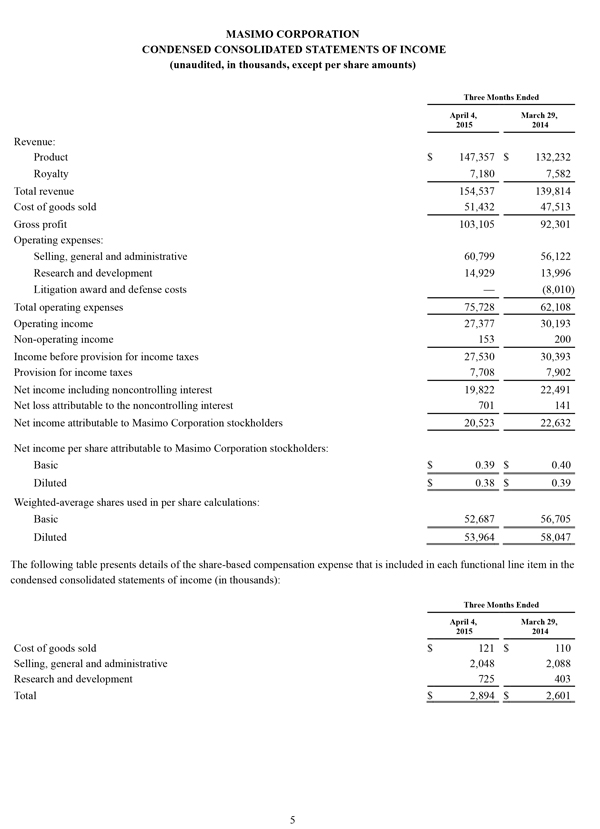
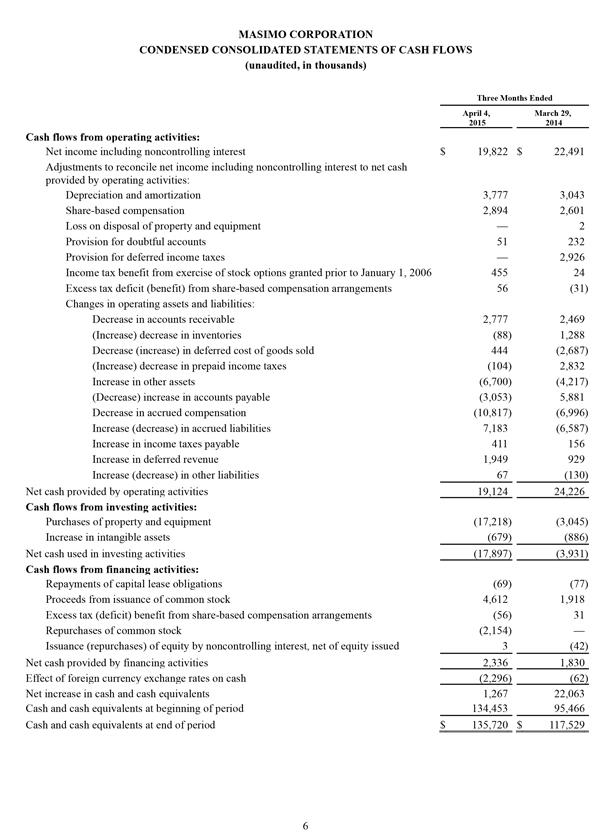
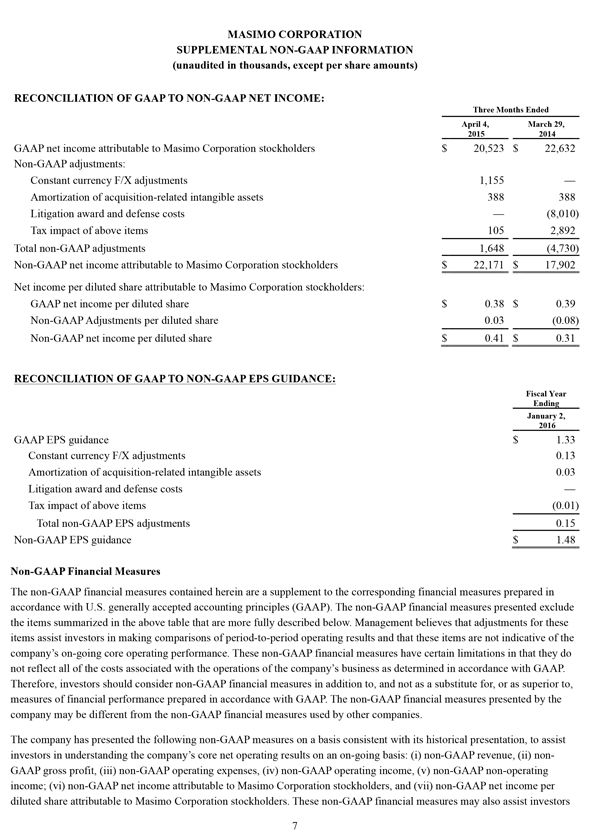

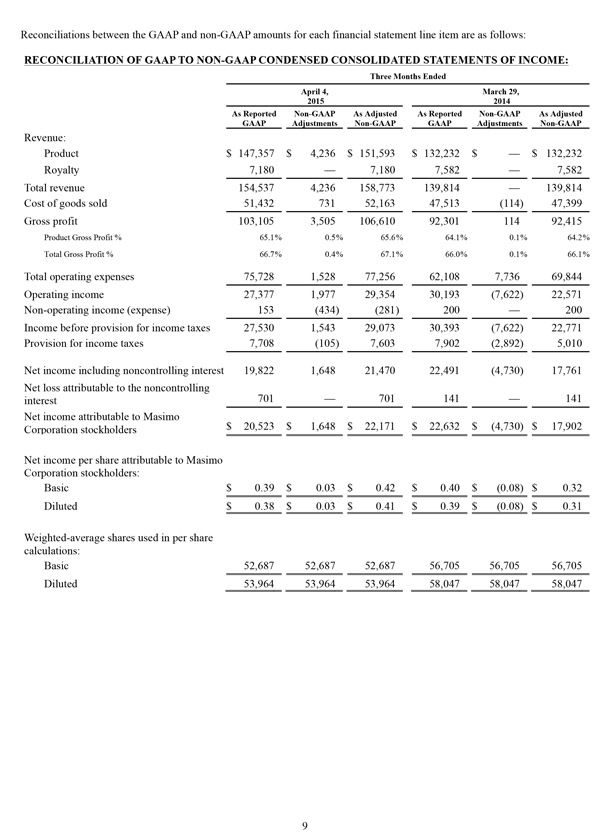
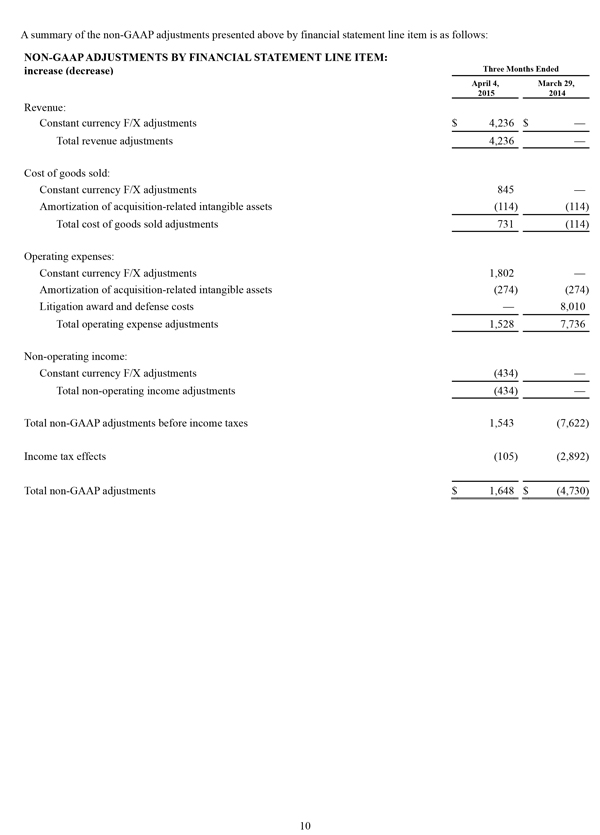


Masimo's MightySat Pulse Oximeter Monitoring Elite Athletes at Vertical Blue Freediving Competition
IRVINE, California & LONG ISLAND, Bahamas – April 29, 2015 – For the first time in freediving history, Masimo (NASDAQ: MASI), maker of breakthrough Measure-through Motion and Low Perfusion™ pulse oximetry, has equipped the Suunto Vertical Blue freediving competition with consumer versions of MightySat® fingertip pulse oximeters.

Dr. Stig Severinsen (left) demonstrates Masimo's MightySat fingertip pulse oximeter with the 2015 Vertical Blue freediving safety team.
Vertical Blue, dubbed "the Wimbledon of freediving" by the New York Times, runs April 27-May 7. The elite event is held each year at Dean's Blue Hole, Long Island, Bahamas – the world's deepest known underwater sinkhole at 663 feet. This year 24 freedivers from 13 countries will take the plunge in three different disciplines: constant weight (CWT), free immersion (FIM) and constant no-fins (CNF).
Before dives, freedivers typically prepare with physical, mental and breath-holding exercises to slow their metabolism, heart rate, breath rate, and the level of carbon dioxide in their bloodstream. Despite these precautions, freedivers risk shallow- and deep-water blackouts. At Vertical Blue, all competitors will be under supervision of a safety team, sponsored by Masimo.
Additionally, Dr. Stig Severinsen, four-time freediving world champion and holder of the Guinness Book of World Records for an underwater breath hold (22 minutes), will monitor blood oxygenation, pulse rate and other parameters throughout the 10-day competition using a MightySat. Severinsen's monitoring will advance the understanding of what event organizers call one of the world's fastest-growing sports.
"The monitoring of these world-class athletes with this new state-of-the-art device should hopefully yield information to be used for improved training and competition protocols," Severinsen said. "The wireless MightySat from Masimo contains advanced technology to deliver reliable, noninvasive measurements on oxygen saturation levels and pulse rate, making it easy to sample and store data even under extreme conditions, such as high and low temperatures and during states of low perfusion."
"Vertical Blue is looking forward to collaborating this year with Masimo, as part of our efforts to be more informed as to the fascinating physiological changes that occur during deep breath-hold dives," said William Trubridge, 15-time freediving champion and founder of Vertical Blue. "Using Masimo equipment, we can collect more data and monitor our athletes throughout the event, with very simple and noninvasive protocols. We're looking forward to seeing the results!"

At Vertical Blue, all competitive freedivers will be under supervision of a safety team, sponsored by Masimo.
MightySat is available in three versions – each provides oxygen saturation (SpO2), pulse rate (PR), and perfusion index (PI) measurements in a compact, battery-powered design with a large color screen that can be rotated for real-time display of the pleth waveform as well as measurements. Features include:
> Signal I.Q.® to assess measurement confidence
> Rugged, lightweight design for operation in challenging environments
> Long battery life–up to 15 hours with two standard AAA alkaline batteries
> Optional Bluetooth LE wireless functionality enables measurement display via a free, downloadable app on iOS and Android mobile devices as well as the ability to trend and communicate measurements
> Optional PVI version for those who want to use their pulse oximeter to evaluate another physiologic dimension. MightySat is the only fingertip pulse oximeter available with Pleth Variability Index (PVI) measurement. (PVI is a measure of the dynamic changes in the perfusion index (PI) that occur during the respiratory cycle.)
> Two-year limited warranty
About Masimo:
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion™ pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements:
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors discussed in the Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Proudly Sponsors Award-Winning Documentary "Personal Gold" During 2015-16 Film Festival Season
Irvine, California – April 23, 2015 – Masimo (NASDAQ: MASI), maker of breakthrough Measure-through Motion and Low Perfusion™ pulse oximetry, announced today that it is the presenting sponsor of the award-winning documentary "Personal Gold: An Underdog Story" for the 2015-16 film festival season.

Heralded as "Miracle on Ice-meets-Moneyball" by Wall Street Journal reporter Reed Albergotti, "Personal Gold" gives a behind-the-scenes experience of four underdog women cyclists who become America's hope for a medal at the 2012 London Olympics after the men's team was banned during the Lance Armstrong drug scandal.
The underfunded women relied on pulse oximetry and other technologies as part of an innovative "Data not Doping" training regimen to do the impossible – win the first U.S. Women's Track Cycling medal in more than 20 years.
The film features a consumer version of Masimo's pulse oximeter, released for sports and aviation.
"I use Masimo pulse oximetry as part of my training and recovery regimen," said U.S. silver medal cyclist Dotsie Bausch. "By tracking my oxygenation and pulse rate, along with numerous other biological and behavioral metrics, I use the data to measure and improve my athletic performance and gauge my recovery."
A private "sneak peek" screening of "Personal Gold" is Friday, April 24, at the Newport Beach Film Festival, followed by a Q-and-A with the filmmakers and one of the cyclists.
#mypersonalgold
@MasimoInnovates
@nbff
About Masimo:
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion™ pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Media Contacts:
Mike Drummond
Masimo
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


U.S. Chamber of Commerce Presents Masimo CEO Joe Kiani with Intellectual Property Champion Award
Irvine, California – April 23, 2015 – The U.S. Chamber of Commerce Global IP Center presented Joe Kiani, founder, CEO and Chairman of the Board of Masimo (NASDAQ: MASI), with the 2015 Life Sciences IP Champion Award at a ceremony commemorating World IP Day in Washington, D.C.

Joe Kiani, founder, CEO of Masimo.
Mr. Kiani has more than 65 patents in his name and more than 575 patents issued or filed by Masimo, in medical technologies related to noninvasive patient monitoring. Mr. Kiani is the first individual in the Life Sciences category to receive this prestigious award. He was selected for his pioneering work in life sciences, his prolific patent portfolio, his passion for innovation, and his advocacy for strong intellectual property protection in the United States and across the globe.
"IP Champion Awards celebrate life-saving cures, amazing inventions, creative content, ground-breaking entrepreneurs, and protection efforts across a range of industries and geographies," Brian Noyes, executive director of strategy and communications at the U.S. Chamber of Commerce's Global IP Center. "IP Champions demonstrate how innovation enriches our lives, and how intellectual property is key to their development."
The Global IP Center noted that the U.S. economy is driven by the need to innovate, create, and develop new ways to serve consumers, and that intellectual property protection plays an essential role. When inventors, researchers, engineers, artists, and entrepreneurs know that their work will be protected and rewarded through strong IP rights – including patents, trademarks, and copyrights – they have the certainty and the incentive to keep the economy-boosting innovations coming.
"I am honored to receive this award. Innovation is not only vital for improving patients' lives but is also the cornerstone of our economy. My company was built on breakthrough innovations that not only made noninvasive pulse oximetry reliable, but made noninvasive monitoring of hemoglobin, carbon monoxide, and many other blood constituents possible. Without the promise of a strong IP system to defend our innovation, we wouldn't have been able to raise the more than $90 million in venture capital financing we needed to break even," said Joe Kiani, founder and CEO of Masimo "However, to be a champion of innovation means so much more to me. In the middle of more than 100 billion galaxies each with billions of stars and all of the the surrounding forces, innovation is crucial for the survival of the human race. To be on the side of a strong and deliberate IP system that fosters innovation, is to be on the right side of history."
The U.S. Chamber of Commerce created the Global Intellectual Property Center to meet the serious criminal and policy threats facing investors, IP-industries, and consumers. Today, the organization is leading a worldwide effort to champion intellectual property rights as vital to creating jobs, saving lives, advancing global economic growth, and generating breakthrough solutions to global challenges. For more information, please visit www.theglobalipcenter.com.

About Masimo:
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion™ pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements:
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: whether Mr. Kiani's receipt of the 2015 IP Champion award will have any material effect on our business, as well as other factors discussed in the Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Announces Patient SafetyNet Series 5000 with Iris Connectivity and MyView
Bringing Connected Care and Enhancing Clinician Workflow from the Operating Room to the Medical and Surgical Unit
Irvine, California – April 13, 2015 – Masimo (NASDAQ: MASI) today announced the debut of Patient SafetyNet Series 5000™ along with Iris™ Connectivity and MyView™ through the Root® patient monitoring and connectivity platform at the HIMSS Annual Conference and Exhibition in Chicago. This series of Patient SafetyNet offers a new level of interoperability designed to enhance clinician workflows, and reduce the cost of care, from operating rooms to medical-surgical units.
Today's medical-surgical units utilize intermittent vital signs spot checks, standalone monitoring systems, and a host of disparate legacy devices, which become isolated stores of valuable patient data. Existing approaches for device interoperability typically require separate hardware, software, and/or network infrastructure, which can clutter the patient room, increase complexity, burden IT management, and increase costs.
A New Level of Data Integration & Workflow Optimization
Masimo's Patient SafetyNet Series 5000 with Iris enables Root to intake data from all devices connected to the patient, acting as an in-room patient monitor and connectivity hub. Alarms and alerts for all devices are seamlessly forwarded to the patient's clinician and all device data are effortlessly documented in the patient's electronic medical record (EMR).
The patient-centric user interface of Patient SafetyNet Series 5000 displays near real-time data from all devices, providing a single unified dashboard of patient information. Having such a holistic view can enable caregivers to more quickly assess patient status allowing for faster clinical decisions.
To simplify documentation of patient data, Root enables clinicians to easily verify and send patient vitals, as well as all connected medical device information data to the EMR directly from Root. Data can also be sent to the EMR periodically. An interface between the Patient SafetyNet Series 5000 Appliance and the hospital ADT system allows clinicians to receive ADT information on Root for positive patient identification at the bedside. Clinicians can also manually enter additional data on the Root device, including temperature, blood pressure, level of consciousness, pain score, and urine output.
Personalized Displays & Reporting with MyView™
MyView is a wireless, presence-detection system that enables clinicians to automatically display customized clinical profiles on Masimo devices, such as Root, Radical-7, and the Patient SafetyNet View Station. When a clinician approaches the device, a clinician-worn MyView badge signals the device to display a preselected set of parameters and waveforms tailored to the individual clinician's preferences.
MyView allows clinicians the ability to consume medical device information in a manner that is most conducive to optimizing their workflow, while the presence mapping data collected by all the Masimo devices can provide information on how clinicians spend time with their patients. This allows nursing leadership and management the opportunity to examine analytical data on patient and clinician interactions to optimize workflows across the unit, hospital, or hospital system.
In 2012, The Joint Commission Sentinel Event Alert on the safe use of opioids in hospitals recommended implementation of better dosing along with continuous oxygenation and ventilation monitoring (instead of spot checks) in post-surgical patients.1 After implementing Masimo SET® and Patient SafteyNet remote monitoring and wireless notification system in a post-surgical floor where only intermittent spot-checking was used before, Dartmouth-Hitchcock Medical Center reduced rapid response activations by 65%* and ICU transfers by 48%,2 and realized $1.48 million in annual opportunity cost savings. Based on the results in this single unit, Dartmouth expanded the system to all seven of its medical-surgical units and had zero patients experience brain injury or death over a five-year period.3
"Medical device interoperability is a key component to improving clinical decision-making, patient safety, and clinician workflow," said Joe Kiani, founder and CEO of Masimo. "Unfortunately many technical and corporate barriers can make achieving interoperability challenging for hospitals. Our goal with Patient SafetyNet Series 5000, Root with Iris, and MyView is to eliminate these barriers and offer innovation that automates patient care with open, scalable, and standards-based connectivity solutions."

1 The Joint Commission Sentinel Event Alert, 2012;49
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. *The calculation of reduced rapid response activations by 65% was based on reduction of rescue events from 3.4 per 1000 discharges to 1.2 per 1000 discharges.
3 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012.
About Masimo:
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com. @MasimoInnovates
Forward-Looking Statements:
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that Masimo Patient SafetyNet can help keep patients safer by noninvasively, continuously measuring and tracking their underlying physiological condition to help hospitals avoid preventable patient deaths and injuries associated with failure to rescue events; risks related to our assumptions regarding the repeatability of clinical results; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.



Three Fire Departments to be Honored for Excellence in Emergency
Medical Services
Fire Service-Based EMS Award Presentation at 27th Annual National Fire and Emergency Services Dinner
WASHINGTON, D.C., and IRVINE, California – April 9, 2015 – The Congressional Fire Services Institute and Masimo (NASDAQ: MASI) will honor three fire departments for best practices and innovative solutions in the delivery of emergency medical services with the Excellence in Fire Service-Based EMS Award. The award presentation takes place on April 16 at the 27th Annual National Fire and Emergency Services Dinner in Washington, D.C.

- Caribou (ME) Fire and Ambulance Department: Improvements in staffing to provide better emergency medical care to patients, and enhanced rehab protocols to insure the health and wellbeing of responding personnel.
- County of Henrico (VA) Division of Fire: Cooperative relationships with the U.S. Military and Department of Defense that have enabled the agency to gain access to equipment and training used by the military to treat soldiers in combat. Through these efforts, Henrico Fire has improved its trauma care.
- City of Scottsdale (AZ) Fire Department: Establishment of an alternate care site at large events to identify and treat alcohol intoxication, so patients could be safely discharged to home and bypass the emergency department.
"CFSI takes great pride in co-sponsoring the Excellence in Fire Service-Based EMS Award with Masimo," said CFSI President Bill Jenaway. "Through this program, we are able to recognize important innovations in fire service-based emergency medical care that other fire departments can adopt to enhance their own emergency medical care systems."
"Through its development of noninvasive medical technologies, including the ability to measure carbon monoxide in the blood, Masimo has a long history of helping first responders monitor themselves and the citizens they protect," said Masimo founder and CEO Joe Kiani. "At this year's Excellence in Fire Service-Based EMS Award, Masimo is honored to help recognize the brave men and women who daily risk their lives and work to improve public safety."
Approximately 1,800 fire and emergency services leaders from across the nation will attend the National Fire and Emergency Services Dinner to pay tribute to the dedication and commitment of the nation's fire and emergency services. Hosted by CFSI, the annual dinner benefits the mission of the nonprofit policy organization, which is designed to educate members of Congress about fire and life safety issues.
First presented in 2011, the Excellence in Fire Service-Based EMS Award has recognized fire departments from across the nation for developing and enhancing the delivery of emergency medical services to address the growing challenges in delivering emergency medical care. By showcasing these practices, the awards program provides ideas for other fire departments to consider implementing, as they seek to improve their fire service-based EMS systems.
About CFSI:
Established in 1989, the Congressional Fire Services Institute (CFSI) is a nonprofit, nonpartisan policy institute that works with members of Congress in promoting fire and life safety issues. Working with other national fire service organizations, the Institute focuses its attention on issues that benefit all first responders. Members of the Congressional Fire Services Caucus, the largest caucus in Congress, look to CFSI for guidance and information to enhance their level of understanding about the challenges and needs of our nation's fire and EMS communities. www.cfsi.org
About Masimo:
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion™ pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Media Contacts:
Bill Webb
CFSI
Phone: (202) 371-1277
Email: bwebb@cfsi.org
Mike Drummond
Masimo
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Illinois Fire Service Institute Is First to Train Firefighters on New Carbon Monoxide Safety Standard
Irvine, California – April 2, 2015 – The world-renowned Illinois Fire Service Institute has become the site of the first program in the nation to train firefighters on new safety standards designed to better protect firefighters from carbon monoxide (CO) poisoning, including use of Masimo's first and only FDA-cleared arterial blood CO monitor, SpCO® technology, to noninvasively monitor CO levels in the blood.
Hosted by the City of Champaign (Ill.) Fire Department in cooperation with the Illinois Fire Service Institute, this program set the course for national training efforts. Hundreds of firefighters from the Champaign Fire Department and across Illinois recently took part in a three-day training course, triggered by the National Fire Protection Association's (NFPA) newly updated Fire Rehabilitation Standard (NFPA 1584), which requires firefighters exposed to smoke at incident scenes and during training to be assessed for CO poisoning.  Randolph Mantooth, a national advocate for CO monitoring and firefighter safety who is recognized for his role as Los Angeles County firefighter/paramedic John Gage in the television show, "Emergency!", delivered the opening remarks for the course. With the support of the Congressional Fire Services Institute and other organizations, Masimo is helping departments and agencies nationwide implement the new standard through peer-reviewed training programs.
Randolph Mantooth, a national advocate for CO monitoring and firefighter safety who is recognized for his role as Los Angeles County firefighter/paramedic John Gage in the television show, "Emergency!", delivered the opening remarks for the course. With the support of the Congressional Fire Services Institute and other organizations, Masimo is helping departments and agencies nationwide implement the new standard through peer-reviewed training programs.
Exposure to CO – a tasteless, odorless, invisible gas – puts firefighters at significant risk, as even mild CO poisoning can cause mental confusion, which can lead to poor decision-making that puts both the exposed firefighter and others in jeopardy.1 CO poisoning also poses long-term health risks. Heart injury is a frequent consequence of moderate to severe CO poisoning,2 while consistent exposure to CO poisoning may cause long-term heart and neurological damage.3 CO poisoning is often misdiagnosed because symptoms are similar to the flu.4
Masimo's rainbow® SET technology provides noninvasive carboxyhemoglobin saturation (SpCO), a measure of CO in the blood, from a single multi-wavelength finger sensor that also provides two other vital signs measurements required by the NFPA 1584 standard, oxygen saturation (SpO2) and pulse rate.
Gary Ludwig, fire chief of the Champaign Fire Department and well-known author and speaker said, "The training that was delivered was invaluable. I've heard nothing but positive comments from the firefighters who took the class, and we were particularly impressed with the ability to noninvasively monitor CO levels at the scene. We all believe that firefighters should go home at the end of their shift. This important class will help us to make sure we are rehabbing and medically monitoring our firefighters during operations."
@champaignfire
SpCO is not intended as a standalone diagnostic test. Laboratory testing should be performed prior to diagnosis.
1 Jakubowski G. FireRescue Magazine. 2004; 22(11):52-55.
2 Henry C. et al. "Myocardial injury and long-term mortality following moderate to severe carbon monoxide poisoning."
JAMA, January 2006, 295(4);398-402.
3 Bledsoe BE. Journal of Emergency Medical Services. 2007; 32:54-59.
4 Varon J. et al. Carbon monoxide poisoning: A review for clinicians. J Emerg Med 1999; 17(1):87-93.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion™ pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com. @MasimoInnovates
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo SpCO will provide an accurate and effective noninvasive method of screening for CO poisoning, risks related to our assumptions of the repeatability of clinical results obtained, and risks related to our assumptions that Masimo SET® pulse oximetry technology improves patient outcomes, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo
mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


New Clinical Study Presented at the International Anesthesia Research Society's Annual Meeting Shows Benefit of Masimo's Oxygen Reserve Index, ORI
Irvine, Calif. – March 30, 2015 – Masimo's (NASDAQ: MASI) announced today that a new clinical study presented at the International Anesthesia Research Society's2015 Annual Meeting showed that Masimo's latest noninvasive patient monitoring parameter, Oxygen Reserve Index™ or ORI™, could help clinicians in the

early detection of an impending desaturation in patients receiving supplemental oxygen.1
Pulse oximetry (SpO2) provides noninvasive and continuous visibility to arterial blood oxygenation in hypoxia and normoxia (normal oxygenation). Clinicians often use arterial oxygen partial pressure (PaO2), which can be intermittent and delayed, to monitor levels of hyperoxia (higher than normal oxygenation). Between invasive sampling, changes in PaO2 can be difficult to assess, and therefore hypoxia or hyperoxia can occur unexpectedly.
ORI, Masimo's 11th rainbow® parameter2, is a relative index of the partial pressure of oxygen in arterial blood (PaO2) in the range of 100 to 200 mmHg. ORI is intended to supplement, not replace, SpO2 monitoring and PaO2 measurements. In the retrospective study conducted at Loma Linda University School of Medicine in Loma Linda, Calif., Dr. Richard Applegate, M.D., and colleagues evaluated the relationship between ORI and PaO2 of 103 patients who underwent surgery in which arterial catheterization was planned.
Researchers included for analysis 1,540 ORI samples using a Masimo Radical-7® Pulse CO-Oximeter®. Regression analysis was used to compare PaO2 from clinically indicated arterial blood gas samples to ORI and calculated changes in ORI (ΔORI) to calculated changes in PaO2 (ΔPaO2).
During a total of 2,377 monitored hours, researchers found that ORI could be calculated about 91.5% of that time, and indicated that ORI was a noninvasive method for measuring moderate hyperoxia (PaO2 was ≥150 mmHg in 96.5% of ORI >0.54, while PaO2 was >100 mmHg for all ORI >0.24).
Investigators noted; "An ORI decrease to near 0.24 may provide early warning of declining PaO2 approaching 100 mmHg," such as during procedures that mandate apnea or one lung ventilation; during difficult intubation; trauma patients with pulmonary contusion or massive resuscitation, and that "the ability to detect PaO2 ≥150 mmHg by ORI >0.54 may prove useful for titrating (fraction of inspired oxygen) FiO2."
Researchers concluded: "For intraoperative PaO2 between 240 and 100 mmHg, decrease in ORI appears to provide a clinically useful indication of falling PaO2 before desaturation," and further stated, "Use of this technology could allow for early detection of impending hypoxia in the critical care setting, particularly in patients with congestive heart failure, which would lead to more rapid treatment and possibly mitigate the need for intubation and mechanical ventilation."
Radical-7® with Root has a CE Mark with the ORI parameter and is not FDA cleared and is not available for sale in the United States.
1. Applegate R, Dorotta I, Applegate P, Andrews G, Olson M, Um M. "Relationship Between Oxygen Reserve Index and Arterial Partial Pressure of Oxygen During Surgery." Proceedings of the International Anesthesia Research Society's 2015 Annual Meeting, March 23, Honolulu, Hawaii, PR03-56-Technology, Computing and Simulation, Equipment Monitoring 5, Coral Ballroom, S-377. 2. 11 parameters include: 1) oxygen saturation (SpO2); 2) Pulse rate; 3) Perfusion index (PI); 4) Pleth Variability Index (PVI); 5) Respiration Rate from the pleth (RRp); 6) Total hemoglobin (SpHb); 7) Oxygen Content (SpOC); 8) Carboxyhemoglobin (SpCO); 9) Methemoglobin (SpMet); 10) Fractional oxygen saturation (SpfO2); 11) Oxygen Reserve Index (ORI)
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using Masimo PVI, risks related to our belief that PVI is an easy-to-use and cost-effective measure for assessing whether patients will benefit from fluid administration, risks related to our assumptions that PVI enables personalized and goal-directed fluid therapy, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo
mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Penn Highlands DuBois Installs Masimo SedLine Brain Function Monitoring
DuBois, Penn., & Irvine, Calif., March 26, 2015 - Penn Highlands DuBois - with one of the best hospital safety scores as reported by the Leapfrog Group – announced today it has selected Masimo's (NASDAQ: MASI) SedLine® brain function monitoring through the Root® patient monitoring and display platform to monitor patients under anesthesia or sedation. Penn Highlands DuBois had been using Bispectral IndexTM (BISTM) technology from Covidien.

"SedLine gives us more data on depths of anesthesia, and the Root technology platform allows us more additional Masimo MOC-9 technology options down the road to expand to fit our critical care monitoring needs," said Aaron DeVallance, MSN, CRNA, Director of Anesthesia at Penn Highlands. "The real-time numbers are faster to display – so it gives us a rapid update on the anesthetic status of our patients, allowing for a faster response time and more timely decisions."
Root offers Masimo's SedLine® brain function monitoring, which provides four channels of EEG to help clinicians better understand the effects of anesthesia on the brain. SedLine measures the effects of anesthesia and sedation by monitoring both sides of the brain's electrical activity. As such, SedLine provides a wealth of EEG-based data, which can help clinicians monitor anesthesia.
<ulrole="presentation" style="width: 100%;">"Time matters, of course, when it comes to clinical decisions," DeVallance added. "But SedLine also offers us cost savings. We're not over-sedating patients, so we're not wasting (anesthesia) gases."
"We are proud that after careful consideration, Penn Highlands DuBois selected Masimo Root with Sedline for its brain functioning monitoring needs," said Dr. Stephen Barker, M.D., Ph.D., Chairman of the Scientific Advisory Board of Masimo. "Sedline can help continuously monitor a patient's level of anesthesia and sedation in the OR and ICU settings, enabling clinicians to monitor anesthesia."
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOCTM), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using Masimo PVI, risks related to our belief that PVI is an easy-to-use and cost-effective measure for assessing whether patients will benefit from fluid administration, risks related to our assumptions that PVI enables personalized and goal-directed fluid therapy, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws
Media Contacts:
John Brennan
Penn Highlands DuBois
(814) 298-0491
jpbrennan@phhealthcare.org
Mike Drummond
Masimo
(949) 297-7434
mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo to Showcase Root® Solutions for the OR and ICU at the International Anesthesia Research Society's 2015 Annual Meeting and International Science Symposium
Irvine, Calif., March 19, 2015 – Masimo (NASDAQ: MASI) announced today that the world's top anesthesiologists will see how Masimo's Root®patient monitoring and connectivity platform is transforming patient care in the operating room and the intensive care unit at the International Anesthesia Research Society's 2015 Annual Meeting and International Science Symposium in Honolulu, Hawaii, March 21-24.
Root integrates Masimo's SET® Measure-through Motion and Low PerfusionTM pulse oximetry and breakthrough noninvasive rainbow® measurements – including continuous, noninvasive hemoglobin (SpHb®) technology – in an integrated, clinician-friendly platform. With a dock for the Radical-7® handheld monitor, an instantly interpretable display, and multiple networking/connectivity options, Root integrates multiple streams of data on an easy-to-read, touch-screen.
Root innovations include:

- MOC-9TM – Flexible measurement expansion through Masimo Open ConnectTM (MOC-9TM) with MOC-9 modules from Masimo or third-party measurement by other companies to expand the platform's measurements and capabilities. New MOC-9 modules in the U.S. will require new 510(k) clearances
- Capnography – ISATM CO2 sidestream module featuring fast warm up time and the innovative and cost-effective NomolineTM sampling line

- IrisTM – Built-in connectivity gateway through IrisTM for standalone devices such as IV pumps, ventilators, hospital beds, and other patient monitors to the EMR
- Wireless functionality – Capable of transmitting information through Bluetooth and Wi-Fi
- Raidus-7TM – The first and only wearable, wireless monitor with Masimo'sbreakthrough rainbow® SET® technology, offering patients continuous monitoring with freedom of movement. Studies have shown that patient mobility is a key factor in more rapid patient recovery.1 Radius-7 allows clinicians to continuously monitor their patients when they are mobile.
Root also offers Masimo's SedLine® brain function monitoring, which provides four channels of EEG to help clinicians better understand the effects of anesthesia on the brain. SedLine measures the effects of anesthesia and sedation by monitoring both sides of the brain's electrical activity. As such, SedLine provides a wealth of EEG-based data, which can help clinicians monitor anesthesia.
At IARS, Masimo will host two events with leading anesthesiologists:
"The Elderly Brain and the Pediatric Brain During General Anesthesia and Emergence: From Neurobiology to Electroencephalography" non-CME symposium is 1:30 p.m., March 21, Coral 1, Hilton Hawaiian Village.
Moderator: Dr. Aryeh Shander, M.D., FCCM, FCCP, chief of the Department of Anesthesiology, Critical Care Medicine, Pain Management and Hyperbaric Medicine at Englewood Hospital & Medical Center in Englewood, N.J.
Presenters:
- Dr. Emery Brown, M.D., PhD, professor of anesthesia at Massachusetts General Hospital/Harvard Medical School;
- Dr. Patrick L. Purdon, PhD, assistant professor of anesthesia, Harvard Medical School; and
- Michael Avidan, MBBCh, professor of anesthesiology and cardiothoracic surgery, Washington University School of Medicine, St. Louis, Mo.
Team Masimo Japan also will host an evening event at 6:30 p.m., March 21, Sheraton-Waikiki.
Presenters:
- Makoto Ozaki, professor and chairman of the Department of Anesthesiology at Tokyo Women's Medical University, will deliver the opening remarks;
- Dr. Emery Brown, M.D., PhD of Massachusetts General Hospital/Harvard Medical School, will lead a special session, "A New Look at Using the EEG to Monitor the Brain States in the Anesthetized Patient";
- Maiko Hasegawa, senior assistant professor, Department of Anesthesiology and Critical Care Medicine at Kagoshima University Medical and Dental Hospital, will offer a clinical report on Root and SedLine.
1 Needham D, Korupolu R, Zanni J, Pradhan P, Colantuoni E, Palmer J, Brower R, Fan E. "Early Physical Medicine and Rehabilitation for Patients With Acute Respiratory Failure: A Quality Improvement Project." Archives of Physical Medicine and Rehabilitation Vol 91, Issue 4, PP 536–542, April 2010
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOCTM), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com .
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using Masimo PVI, risks related to our belief that PVI is an easy-to-use and cost-effective measure for assessing whether patients will benefit from fluid administration, risks related to our assumptions that PVI enables personalized and goal-directed fluid therapy, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws
Media Contact:
Mike Drummond
Masimo
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Korean Regulators Clear Masimo PVI® for Clinical Use
Neuchatel, Switzerland – February 26, 2015 – Masimo (NASDAQ: MASI) announced today that the Republic of Korea's Ministry of Food and Drug Safety (MFDS) and the Korean Health Technology Assessment (HTA) group have cleared Masimo's noninvasive PVI® – a measure of the dynamic changes in the perfusion index that occur during one or more complete respiratory cycles – for clinical use.
Korean regulators determined that PVI is an effective, noninvasive way to predict fluid responsiveness in mechanically ventilated patients. Regulators concluded PVI has higher accuracy of diagnosis compared to static indices (central venous pressure, pulmonary capillary wedge pressure), and similar accuracy diagnosis with dynamic indices (cardiac output variability, pulse pressure variability, blood volume amplitude variability, systolic pressure variability). They stated:
"Pleth Variability index (PVI) measurement measures pleth variability non-invasively with the sensor on the skin of patients, so that there is no issue for safety.
"Therefore, PVI is safe and effective as (an) alternative non-invasive PVI measurement to predict fluid responsiveness of machine respiration patients who need fluid supply."
Clinicians commonly use fluid administration to improve haemodynamics before, during and after surgery. Assessment of fluid responsiveness – the ability of the circulation system to increase cardiac output in response to volume expansion – is essential to guide fluid therapy and optimize preload.1 Too little fluid administration can result in low perfusion in peripheral tissue, but too much fluid administration can result in patients failing to respond to any amount of volume expansion,2,3 as well as fluid overload postoperatively.4,5
This clearance by Korean regulators is similar to the decision of the French Society for Anaesthesia and Intensive Care (SFAR), which in 2013 added PVI to guidelines for optimal haemodynamic management during surgery.6 Similar to Korean authorities, SFAR recommends the use of "dynamic" parameters such as PVI that measure variations over the respiratory cycle.
"We're excited to see another major country approve for clinical use another one of our important noninvasive, physiological measurements," said Masimo founder and CEO Joe Kiani. "From PVI, to our rainbow measurements that include noninvasive haemoglobin, carbon monoxide, and our newest noninvasive parameter, Oxygen Reserve Index or ORI, that can help clinicians optimize oxygenation before and during prolonged intubation, Masimo continues to innovate ways to improve patient outcomes and reduce the cost of care by advancing and creating new noninvasive measurements."
PVI is available with any Masimo SET® or rainbow® sensor.
1 Cannesson M: Arterial pressure variation and goal-directed fluid therapy. J Cardiothorac Vasc Anesth 24:487-497,2010.
2 Brandstrup B, TonnesenH, Beier-HolgersenR, etal: Effects of intravenous fluid restriction on postoperative complications: Comparison of two perioperative fluid regimens: A randomized assessor-blinded multicenter trial. AnnSurg 238:641-648,2003.
3 Marik PE, Cavallazzi R, Vasu T, etal: Dynamic changes in arterial wave form derived variables and fluid responsiveness in mechanically ventilated patients: A systematic review of the literature. Crit Care Med 37:2642-2647,2009.
4 Kita T, Mammoto T, Kishi Y. Fluid management and postoperative respiratory disturbances in patients with transthoracic esophagectomy for carcinoma. J Clin Anesth. 2002;14(4):252–6.
5 Brandstrup B, Tonnesen H, Beier-Holgersen R, Hjortso E, Ording H, Lindorff-Larsen K, Rasmussen MS, Lanng C, Wallin L, Iversen LH, Gramkow CS, Okholm M, Blemmer T, Svendsen PE, Rottensten HH, Thage B, Riis J, Jeppesen IS, Teilum D, Christensen AM, Graungaard B, Pott F, Danish Study Group on Perioperative Fluid T. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238(5):641–8. doi:10.1097/01.sla.0000094387.50865.23.
6 Vallet B., Blanloeil Y., Cholley B., Orliaguet G., Pierre S., Tavernier B. "Strategy for perioperative vascular filling - Guidelines for perioperative haemodynamic optimization." Experts' Formalized Recommendations, French Society of Anaesthesia and Intensive Care (SFAR), Validation by the administrative council of SFAR on 19 October 2012.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOCTM), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using Masimo PVI, risks related to our belief that PVI is an easy-to-use and cost-effective measure for assessing whether patients will benefit from fluid administration, risks related to our assumptions that PVI enables personalized and goal-directed fluid therapy, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.



CFSI Announces Masimo as Co-Sponsor of the Excellence In Fire Service-Based EMS Award
(WASHINGTON, D.C., and IRVINE, Calif.) – Feb. 20, 2015 –The Congressional Fire Services Institute has announced Masimo (NASDAQ: MASI) as the new co-sponsor of the Excellence in Fire Service-Based EMS Award. CFSI said Masimo will bring a new level of recognition to the awards program based on its reputation for product innovations for fire departments across the nation.
Established in 2010, the Excellence in Fire Service-Based EMS Award honors fire departments in three categories: career, volunteer and combination. The criteria place emphasis on innovations in emergency medical care.
"We are looking for departments that are forward-thinking in their approach to EMS – departments that are developing and embracing change to adapt to the growing demand for pre-hospital emergency medical care," said CFSI Executive Director Bill Webb. "We found a partner in Masimo that shares these ideals, which is why we welcome them as a new co-sponsor of the awards program."
"Masimo has always valued the heroic work of our nation's firefighting and EMS professionals," said Masimo founder and CEO Joe Kiani. "We are honored to partner with the Congressional Fire Services Institute to recognize leaders who are using the latest technologies to improve the health and safety of their fellow first responders."
The presentation of the 2015 awards is April 16 at the 27th Annual National Fire and Emergency Services Dinner in Washington, D.C. Sponsored by the Congressional Fire Services institute, the dinner is part of the National Fire and Emergency Services Symposium hosted annually by CFSI. More than 2,000 state and national fire service leaders are expected to attend this year's program. Over the two-day event, they will attend seminars featuring federal officials and national fire service leaders who will discuss a broad range of federal fire service issues. Guests will also schedule time with their members of Congress to solicit their support for federal legislation and programs benefiting the fire and emergency services.
To apply, visit Excellence in Fire Service-Based EMS Awards Program. The deadline for submission is March 16, 2015. A representative of each of the recipient departments will receive a trip to Washington, D.C., for the award presentations.
CFSI also acknowledges its thanks to the International Association of Fire Chiefs, International Association of Firefighters, National Fire Protection Association and the National Volunteer Fire Council for providing representatives to serve on the selection committee.
About CFSI:
Established in 1989, the Congressional Fire Services Institute (CFSI) is a nonprofit, nonpartisan policy institute that works with members of Congress in promoting fire and life safety issues. Working with other national fire service organizations, the Institute focuses its attention on issues that benefit all first responders. Members of the Congressional Fire Services Caucus, the largest caucus in Congress, look to CFSI for guidance and information to enhance their level of understanding about the challenges and needs of our nation's fire and EMS communities.www.cfsi.org
About Masimo:
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion™ pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Media Contacts:
Bill Webb
CFSI
Phone: (202) 371-1277
Email: bwebb@cfsi.org
Mike Drummond
Masimo
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.


Masimo Reports Fourth Quarter and Full Year 2014 Financial Results
Q4 2014 Highlights (compared to Q4 2013):
- Product revenue rose 14% to $153.9 million
- Total revenue, including royalties, rose 14% to $161.8 million
- Masimo rainbow® revenue declined 4% to $14.1 million
- SET® and rainbow® SET® unit shipments were 44,100
- Earnings per share was $0.40 versus $0.16 in the year-ago period
FY 2014 Highlights (compared to 2013):
- Product revenue rose 8% to $556.8 million
- Total revenue, including royalties, rose 7% to $586.6 million
- Masimo rainbow® revenue rose by 6% to $51.8 million
- SET® and rainbow® SET® unit shipments were 171,000
- Earnings per share was $1.30 versus $1.02 in the year-ago period
Irvine, California, February 17, 2015 - Masimo (NASDAQ: MASI) today announced its financial results for the fiscal fourth quarter and full fiscal year ended January 3, 2015.
Fourth quarter 2014 product revenues rose 14% to $153.9 million, compared to $134.7 million for the fourth quarter of fiscal year 2013, and total revenue, including royalties, rose 14% to $161.8 million, up from $142.4 million for the fourth quarter of fiscal year 2013. The unfavorable effect of foreign currency movements adversely impacted fourth quarter product revenues by approximately $3.4 million.
The company's worldwide direct product revenue in the fourth quarter of 2014 rose by 14% compared to the same period in 2013 and represented 86% of total product revenue. OEM sales, which accounted for 14% of total product revenue, rose by 18% compared to the same period in 2013. Revenue from sales of Masimo rainbow® products declined by 4% to $14.1 million in the fourth quarter of 2014, compared to $14.8 million in the prior year period.
Net income for the fourth quarter of 2014 was $21.2 million, or $0.40 per diluted share, compared to net income of $9.3 million, or $0.16 per diluted share, in the fourth quarter of 2013. Fourth quarter 2013 results were negatively impacted by $0.15 per diluted share charges related to selected inventory and equipment write-downs, as well as an arbitration award ruling that was subsequently vacated on appeal and reversed in the first quarter of 2014. During the fourth quarter of 2014, the company shipped approximately 44,100 SET® pulse oximetry and rainbow® Pulse CO-Oximetry™ units, excluding handheld units. Masimo estimates its worldwide installed base as of January 3, 2015 to be 1,313,000 units, up 9% from 1,205,000 units as of December 28, 2013.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "We are happy that the fourth quarter product revenue growth reflected a continuation of the recovery in our core business that began in the third quarter. I am also happy that our value engineering efforts are, as we expected, continuing to result in higher sequential gross profit margins. Both of these factors, coupled with our continued focus on overall operating expense control, have begun to show the operating leverage opportunity that exists within our business model."
As of January 3, 2015, Masimo's cash and cash equivalents were $134.5 million, compared to $95.5 million as of December 28, 2013. During the fourth quarter, the company repurchased 27,659 shares of stock for $0.6 million, resulting in total 2014 stock repurchases of approximately 4.5 million shares for $102.5 million.
2015 Financial Guidance
Masimo today is providing 2015 financial guidance. Due to the significant movement in foreign exchange rates over the last four months, and the assumption that those rates will continue throughout 2015, Masimo is, for the first time, providing an estimate of the impact of these foreign exchange rates on its 2015 GAAP financial guidance. Masimo expects fiscal 2015 GAAP total revenues to be approximately $605 million. Masimo expects fiscal 2015 GAAP total product revenues to be $577 million, including an estimated $20 million revenue reduction due to unfavorable 2015 foreign exchange rate assumptions impacting product revenues compared to 2014 actual foreign exchange rates. In addition, Masimo expects approximately $28 million in fiscal 2015 royalty revenues. Masimo also expects fiscal 2015 GAAP earnings per diluted share of approximately $1.30, including an estimated $0.15 reduction due to the more unfavorable 2015 foreign exchange rate assumptions impacting revenues, cost of sales and operating expenses compared to the 2014 actual foreign exchange rates. Masimo will provide additional financial information during the conference call today. Each of the components of Masimo's guidance set forth above is an estimate only and actual performance could differ.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the call will be available online from the investor relations page of the company's website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 76075657. After the live webcast, the call will be available on Masimo's website through March 10, 2015. In addition, a telephonic replay of the call will be available through March 3, 2015. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 76075657.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through-Motion and Low-Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our expectations for full fiscal year 2015 total, product and royalty revenues and GAAP earnings per share; estimates regarding the impact of foreign exchange rates on our financial performance for full fiscal year 2015; statements regarding the recovery in our core business; statements regarding higher sequential gross profit margins and expense control; our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies and reduce the cost of care; and demand for our technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: actual foreign currency exchange rates in fiscal year 2015; our dependence on Masimo SET® and Masimo rainbow® SET® products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the amount and type of equity awards that we may grant to employees and service providers in the future; our ongoing litigation and related matters; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Media Contacts:
Mike Drummond
Masimo
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Investor Contacts:
Eli Kammerman
Phone: (949) 297-7077
Email: ekammerman@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


National Fire Protection Association Issues Updated Standard Requiring Firefighters Exposed to Fire Smoke to be Assessed for Carbon Monoxide Poisoning at Incident Scenes and During Training
Irvine, California – February 12, 2015 – Masimo (NASDAQ: MASI), the inventor of Masimo SET® Measure-Through Motion and Low Perfusion™ pulse oximetry and rainbow® Pulse CO-Oximetry, announced today that the National Fire Protection Association (NFPA) released an updated Fire Rehabilitation Standard (NFPA 1584) requiring firefighters exposed to smoke at incident scenes and during training to be assessed for carbon monoxide (CO) poisoning.
NFPA's consensus codes and standards serve as the worldwide authoritative source on fire prevention and public safety.
The revised standard, effective now, requires that firefighters who are "exposed to fire smoke shall be assessed for carbon monoxide poisoning." The standard further states in Annex A that, "Any firefighter exposed to CO or presenting with headache, nausea, shortness of breath, or gastrointestinal symptoms... should be assessed for carbon monoxide poisoning."
The previous version (2008) of the standard did not include assessment of CO poisoning as a requirement. The new requirement reflects a growing consensus on the risks of CO, the difficulty of assessing CO through signs and symptoms alone, the availability of portable CO monitoring devices, and the benefits of assessing CO poisoning at the scene and during training.
Masimo's rainbow® SET technology provides noninvasive carboxyhemoglobin saturation (SpCO®), a measure of CO in the blood, from a single multi-wavelength finger sensor that also provides two other vital signs measurements required by the NFPA 1584 standard, oxygen saturation (SpO2) and pulse rate.
With the support of the Congressional Fire Services Institute and other organizations, Masimo will expand its efforts to help departments and agencies nationwide implement the new standard.
Gary Ludwig, fire chief of the Champaign (Ill.) Fire Department and well-known author, speaker, and consultant said, "There is nothing more important in our profession than firefighter safety. The new 1584 standard builds on the older standard and more comprehensively addresses medical monitoring and carbon monoxide poisoning of the firefighter. I am excited to see this updated standard and that Masimo is at the forefront of making sure firefighters go home at the end of their shifts."
"Emergency service rehabilitation has been found to be a critical component of firefighter physical and mental well-being," said Dr. William F. Jenaway, president of the Congressional Fire Services Institute and 40-plus year member of the Pennsylvania Fire Service. "The use of rehabilitation including medical monitoring and assessment of carbon monoxide poisoning is no longer 'a nice thing to do,' it is a critical component of firefighter safety and health."
Joe Kiani, founder and CEO of Masimo, stated: "We applaud NFPA for making assessment of carbon monoxide poisoning a requirement. We are proud that our rainbow Pulse CO-Oximetry technology can play a role in helping meet this standard by allowing the noninvasive monitoring of SpO2, pulse rate, and SpCO, and in increasing safety for our heroic public servants."
SpCO is not intended as a standalone diagnostic test. Laboratory testing should be performed prior to diagnosis.
About NFPA
A worldwide leader in providing fire, electrical, building, and life safety to the public since 1896, NFPA's mission is to reduce the global burden of fire and other hazards on the quality of life by providing and advocating consensus codes and standards, research, training, and education. NFPA's 300 codes and standards influence every building, process, service, design, and installation in the U.S. and many other countries. With a membership of more than 81,000 and more than 80 national trade and professional organizations, NFPA is the authority on fire, electrical, and building safety. Copies of the National Fire Protection Association (NFPA) Section 1584, Standard on the Rehabilitation Process for Members During Emergency Operations and Training Exercises, are now available through the NFPA.
About Masimo
Masimo (NASDAQ: MASI) is a global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion™ pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com. @MasimoInnovates
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo SpCO will provide an accurate and effective noninvasive method of screening for CO poisoning, risks related to our assumptions of the repeatability of clinical results obtained, and risks related to our assumptions that Masimo SET® pulse oximetry technology improves patient outcomes, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.



Inova Fair Oaks Hospital Casts Masimo Patient SafetyNet™ to Help Improve Patient Outcomes and Reduce Costs
FAIRFAX, Va., &IRVINE, Calif. – January 15, 2015 – Masimo (NASDAQ: MASI) and Inova Fair Oaks Hospital – listed among the 2014 Truven Health Analytics Top 100 Hospitals® for delivering the best care in its community – announced that the hospital has installed Masimo Patient SafetyNet™, a remote monitoring and clinician notification system shown to keep patients safer while reducing cost of care.1
Inova Fair Oaks Hospital embraces the patient-safety guidelines of leading health organizations, including the Anesthesia Patient Safety Foundation, the Joint Commission, and the Institute for Safe Medication Practices (ISMP), which recommend continuous oxygenation and ventilation monitoring in patients receiving opioid-based pain medications.2-4 Opioid analgesics are associated with adverse effects and cause respiratory depression in 0.50%, or 25 of every 5,000 post-surgical patients.5-9
Patient SafetyNet has been clinically shown to reduce preventable and costly rescue events, transfers to intensive care units, and deaths related to opioid-induced respiratory depression.1,10 Patient SafetyNet combines the performance of Masimo SET® pulse oximetry – the enabler of reliable oxygenation and pulse rate monitoring on medical/surgical floors – with ventilation monitoring and wireless clinician notification that can help ensure patients' safety by noninvasively and continuously measuring and tracking their underlying physiological conditions and changes that signal declining health status in real-time. When changes occur in the measured values, which may indicate deterioration in the patient's condition, the system automatically sends wireless alerts directly to clinicians – prompting a potentially lifesaving response to the patient's bedside.
"Masimo Patient SafetyNet provides an additional layer of patient protection by offering our staff an extra set of 'eyes' on the patient at all times even when a clinician isn't in the room," said G. Michael Lynch, M.D., Inova Fair Oaks Hospital Chief Medical Officer. "Masimo SET pulse oximetry also has the additional benefit of reducing false alarms, even during challenging conditions of patient movement or low perfusion, giving our staff greater confidence that our patients are receiving the best care possible."
1 Taenzer A.H., Pyke J.B., McGrath S.P., Blike G.T. Anesthesiology. 2010 Feb;112(2):282-7
2 Stoelting RK et al. APSF. 2011.
3 Joint Commission Sentinel Event Alert. Issue 49. August 8, 2012.
4 https://www.ismp.org/newsletters/acutecare/articles/20090312.asp
5 Vila H Jr, Smith RA, Augustyniak MJ: The efficacy and safety of pain management before and after implementation of hospital-wide pain management standards: Is patient safety compromised by treatment based solely on numerical pain ratings? Anesthesia and Analgesia, 2005;101:474-80.
6 Office of Applied Studies, Substance Abuse and Mental Health Services Administration. Substance abuse treatment admissions involving abuse of pain relievers: 1998 and 2008, http://oas.samhsa.gov/2k10/230/230PainRelvr2k10.cfm (accessed October 28, 2011).
7 McPherson ML: Strategies for the management of opioid-induced adverse effects. Advanced Studies in Pharmacy, 2008;5(2):52-57.
8 Jarzyna D, et al.: American Society for Pain Management Nursing guidelines on monitoring for opioid-induced sedation and respiratory depression. Pain Management Nursing, 2011;12(3): 118-145
9 Pasero C, M McCaffery: Pain assessment and pharmacologic management. Chapter 12 – Key Concepts in Analgesic Therapy, and Chapter 19 – Management of opioid-induced adverse effects. St. Louis, Mosby Elseveir, 2011
10 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring - The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012.
About Inova
Inova is a not-for-profit health care system located in the Washington, D.C. metropolitan area, serving over two million people with over 1,700 licensed beds based in Northern Virginia. Inova consists of five hospitals including the area's only Level 1 Trauma Center and Level 4 Neonatal Intensive Care unit. Inova encompasses many health services including the nationally and internationally recognized Inova Heart and Vascular Institute (IHVI), Inova Translational Medicine Institute (ITMI) on genomics, Inova Neuroscience Institute and Inova Children's Hospital. Inova's mission is to improve the health of the diverse community it serves through excellence in patient care, education and research. More information and statistics about Inova is at www.inova.org.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that all patients at Inova Fair Oaks Hospital will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo Patient SafetyNet can help keep patients safer by noninvasively, continuously measuring and tracking their underlying physiological condition to help hospitals avoid preventable patient deaths and injuries associated with failure to rescue events; risks related to our belief that Masimo SET virtually eliminates false alarms and increases a clinician's ability to detect life-threatening events; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Amy Richards
Inova Fair Oaks Hospital
Phone: (703) 391-3390
Email: Amy.Richards@inova.org
Mike Drummond
Masimo
Phone: (949) 297-7434
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo


Masimo Unveils MightySat™ at Consumer Electronics Show - First Fingertip Pulse Oximeter with Masimo SET® Measure-through Motion and Low Perfusion™ Technology
Visit Booth 73711 at CES in Las Vegas to Witness Death-Defying and Olympic-Level Demonstrations Showing How MightySat Delivers Accurate Measurements When Other Pulse Oximeters Fail
Irvine, California — January 5, 2015 — Masimo (NASDAQ:MASI) today announced the MightySat fingertip pulse oximeter for personal use. MightySat provides accurate oxygen saturation and pulse rate measurements when other pulse oximeters can fail1 and is ideal for those who want reliable measurements even under extreme conditions.

MightySat™ fingertip pulse oximeter provides oxygen saturation(SpO2), pulse rate (PR), perfusion index (PI), and pleth variability index (PVI®).
Oxygen saturation (the percentage of arterial blood that is bound with oxygen) and pulse rate (the number of times the heart beats per minute) provide an indication of lung and heart efficiency and increasingly are being measured with fingertip pulse oximeters for personal use. However, until now no fingertip pulse oximeter has been available with Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry – the same technology used on more than 100 million patients a year in leading hospitals worldwide. Now, instead of guessing whether their pulse oximeter is providing accurate measurements, people can rely on MightySat with Masimo SET® technology – shown in more than 100 independent and objective studies to outperform all other pulse oximetry technologies with dramatically fewer false measurements during motion and low perfusion (or low blood flow) to the finger.1
MightySat is available in three versions – each of which provides oxygen saturation (SpO2), pulse rate (PR), and perfusion index (PI) measurements in a compact, battery-powered design with a large color screen that can be rotated for real-time display of the pleth waveform as well as measurements. Optional Bluetooth wireless functionality enables measurement display via a free, downloadable app on iOS and Android mobile devices as well as the ability to trend and communicate measurements. And for those who want to use their pulse oximeter to evaluate another physiologic dimension, MightySat is the only fingertip pulse oximeter available with the optional Pleth Variability Index (PVI®), a measure of the dynamic changes in the PI that occur during one or more complete respiratory cycles.2
Other standard features include:
- Signal I.Q.® to assess measurement confidence
- Rugged, lightweight design for operation in challenging environments
- Long battery life–up to 15 hours with two standard AAA alkaline batteries
- Two-year limited warranty
Elite athletes are among those who trust Masimo SET® pulse oximetry to help them achieve peak performance. Stig Severinsen, Ph.D. in medicine, a four-time World Champion freediver and owner of multiple Guinness World Records, including history's longest breath-hold of 22 minutes, will demonstrate MightySat while holding his breath in a coffin-sized water tank during the CES trade show in Las Vegas, Jan. 6-9, Booth 73711, Tech West, Sands Expo.
Also at the Masimo booth will be Dotsie Bausch, a seven-time USA Cycling National Champion, two-time Pan-American Champion, and silver medalist in team pursuit cycling at the 2012 London Olympics, who will perform her Olympic-level training, demonstrating Masimo SET® technology measurements.
"Training for extreme records under extreme conditions is always a huge challenge," said Severinsen, who was the subject of a recent National Geographic television special, when he used Masimo technology prior to freediving inside an iceberg. "In such situations it is of great value to be able to perform noninvasive and accurate measurements of my heart rate and oxygen saturation levels with a state-of-the-art device.
"I would recommend Masimo's MightySat to anyone interested in health and fitness – understanding what goes on inside your body is paramount to improving performance," he added.
Also at CES, win autographed books, consumer medtech devices and other prizes by entering Masimo's social media picture contest:
- Post to your social networks a picture of you from Booth 73711 Tech West, Sands Expo, with Stig Severinsen and/or Dotsie Bausch
- Use both hashtags #CES2015 and #Masimo
Winners will be announced throughout the day.
MightySat is available for purchase on Amazon U.S. and Amazon U.K. Please visit www.masimopersonalhealth.com for purchasing information.
In the U.S., MightySat is intended for sports and aviation use; not intended for medical use. Android is a trademark of Google Inc.
1 Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers".JClinAnesth. 2012 Aug;24(5):385-91. All clinical studies are available at www.masimo.com
2 The utility of PVI is unknown at this time and requires further clinical studies. Technical factors that may affect PVI include probe position and patient motion.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors related to MightySat, including our belief in the breakthrough ability of Masimo SET® pulse oximetry to measure-through motion and low perfusion; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo
Phone: (949) 297-7434
Mobile: (949) 648-2269
Email: mdrummond@masimo.com
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo
| https://psmf.org/ |
