TECHNOLOGY
rainbow Acoustic
Monitoring®
Noninvasive, Continuous Monitoring of Acoustic Respiration Rate (RRa®)
Designed to capture the sounds of breathing in a patient’s airway, rainbow Acoustic Monitoring converts acoustic patterns into breathing cycles to calculate respiratory rate.
- Applied comfortably on the neck or chest to capture the sound of breathing
- Hear a patient’s breathing remotely from a Patient SafetyNet™* view station
- Easily monitor respiration rate in fragile neonates and pediatric patients
The Need for Continuous Respiration Monitoring
Respiratory rate (RR) is one of the most sensitive markers of patient
condition and a core aspect of multiple clinical assessment tools.1 It
provides information on clinical deterioration, predicts cardiac arrest, and
supports the diagnosis of severe pneumonia.2
In fact, respiratory conditions are the most common reason for admission
to a neonatal unit in both term and preterm infants.3 And because
neonates are among the most fragile patients, the need for continuous
monitoring without discomfort or irritation is paramount.4
A Gentle Approach: Acoustic Respiration Rate (RRa)
With its streamlined sensor size and weight, along with an adhesive ideal
for use on delicate skin, RRa offers a gentle approach to measuring
respiration rate, prioritizing patient comfort without sacrificing reliability.
Monitoring RRa alongside SpO2 and other physiological parameters on a
single Masimo Pulse CO-Oximeter® can help facilitate more informed
assessment of the most fragile patients.
How Does Acoustic Respiratory Monitoring Work?
Using acoustic signal processing, rainbow Acoustic Monitoring captures the vibrations caused by a patient’s airflow via a sensor worn comfortably on the chest or neck. These vibrations are displayed as a waveform and converted into breath cycles to calculate acoustic respiration rate (RRa), a numeric value.
To help streamline workflows, RRa can be accessed remotely from a Patient SafetyNet view station.
Watch the video to learn more.

Clinical Evidence
“Acoustic monitoring has the potential to increase the safety of pediatric patients by providing a reliable and accurate method for the continuous monitoring of respiration rate.”5
-
Case Example
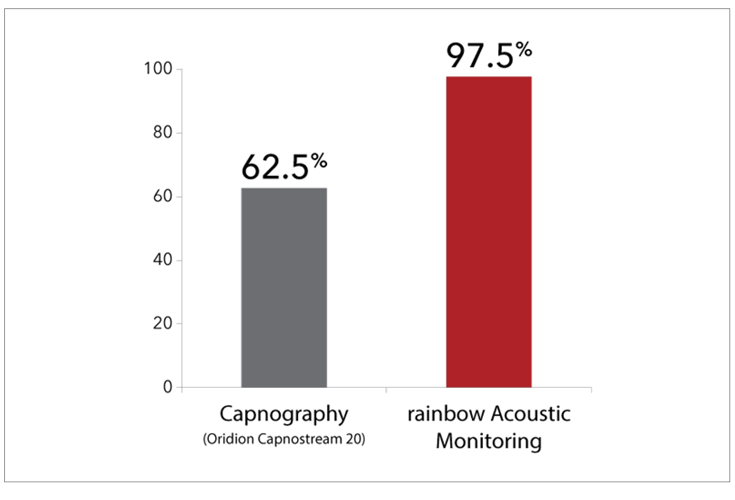
Study Comparing RRa to Other Methods in Pediatric Patients
Pediatric Patient Tolerance
In a study of 40 pediatric patients (12 months to 18 years of age) undergoing post-anesthesia care, in which researchers compared acoustic respiration rate monitoring using RRa to nasal capnography, impedance pneumography, and a reference method (counting breaths), researchers found that the difference in bias and precision between RRa and capnography was not significant, but that 97.5% of the patients (39) demonstrated good tolerance of the acoustic sensor, whereas 62.5% (25) demonstrated good tolerance of the nasal cannula. The researchers concluded, “Continuous respiration rate measurement from noninvasive, acoustic monitoring showed good agreement with nasal capnography, but was much better tolerated in post-surgical pediatric patients.”5
rainbow Acoustic Monitoring Solutions
Explore the components of the rainbow Acoustic Monitoring platform.

rainbow Acoustic® Sensors
Acoustic Monitoring Sensors
Noninvasive, continuous monitoring of acoustic respiration rate (RRa®)
RAM® Dual Cables
rainbow Acoustic Monitoring® Dual Cables
Monitor acoustic respiration rate (RRa®) and rainbow SET® parameters simultaneously
Resources
Need more information? Below are key resources
about rainbow Acoustic Monitoring with RRa.
Schedule an Evaluation
See how RRa can make a difference in your healthcare organization. Please fill out the following information and a Masimo representative will be in touch with you.
References
Philip KE et al. J Clin Monit Comput. 1 2015;29(4):455-60.
Nicolò A et al. Sensors (Basel). 2020;20(21):6396.
Pramanik AK et al. Pediatr Clin North Am. 2015;62:453 -469
Abbas et al. BioMedical Engineering OnLine. 2011;10:93.
Patino M et al. Pediatric Anesthesia. 2013;12:1166-1173.
For professional use. See instructions for use for full prescribing information including indications,contraindications, warnings and precautions. Caution: Federal (USA) law restricts this device to sale by or on the order of a physician.
*The use of the trademark PATIENT SAFETYNET is under license from University HealthSystem Consortium.
PLCO-007683/PLM-11608D-0225